<img src="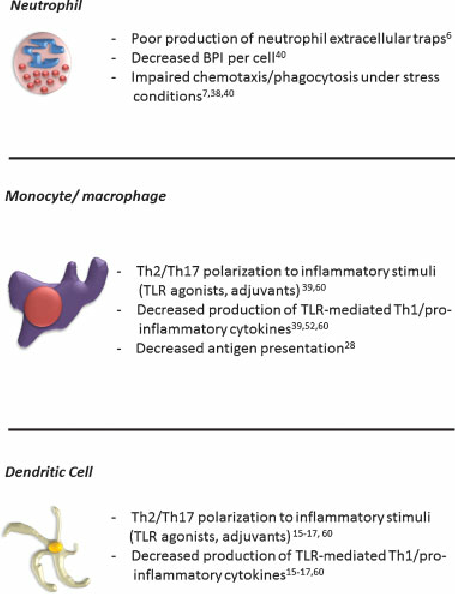 "/>
"/>
<h2>Abstract</h2>
Newborns are at increased risk of infection due to genetic, epigenetic, and environmental factors. Herein we examine the roles of the neonatal innate immune system in host defense against bacterial and viral infections. Full-term newborns express a distinct innate immune system biased towards TH2/TH17-polarizing and anti-inflammatory cytokine production with relative impairment in TH1-polarizing cytokine production that leaves them particularly vulnerable to infection with intracellular pathogens. In addition to these distinct features, preterm newborns also have fragile skin, impaired TH17-polarizing cytokine production and deficient expression of complement and of antimicrobial proteins and peptides (APPs) that likely contribute to susceptibility to pyogenic bacteria. Ongoing research is identifying APPs, including bacterial/permeability-increasing protein and lactoferrin, as well as pattern recognition receptor (PRR) agonists that may serve to enhance protective newborn and infant immune responses as stand alone immune response modifiers or vaccine adjuvants.
<h2>Introduction </h2>
Infection of newborns and infants, including bacterial sepsis, is a major health care issue with an annual global mortality in excess of one million lives. Indeed, infection is the leading cause of mortality among infants in the first days of life1, 2. The incidence of infection can vary widely depending on gestational age and time of onset, with severe infection having higher incidence and mortality in very low birth weight (VLBW) premature neonates, within the first three to seven days of life3, 4. The economic burden of caring for and hospitalizing these infected infants is considerable, and is estimated at approximately $700 million in the US alone5 . Infection during the neonatal and infant period has also been recognized as an international issue. In an attempt to counter and improve health conditions for infants globally, the United Nations has outlined a series of eight Millenium Development Goals to decrease by 2/3 the mortality of children under the age of five by 20155 .
The purpose of this review is to examine current evidence regarding the role of the innate immune response during neonatal infection and highlight it’s relevance to the practicing clinician. We will discuss distinct aspects of neonatal innate immune signaling pathways in response to infection. Next we explore current insights into efforts to prevent neonatal infection using immune adjuvants and vaccines. Lastly, we will examine future directions and goals in the field and elaborate on exciting new therapeutic possibilities. Prior to our discussion of the role of neonatal-specific innate immunity in neonatal infection, a very focused overview of pertinent immunology will likely be helpful to effectively communicate our objectives. For a more complete review of these topics, the reader is also referred to recent reviews6–9 .
The first concept we will discuss relates to innate immune function and pathogen detection. Innate immune cells recognize pathogens via pattern recognition receptors (PRRs), of which the most well-studied are the Toll-like receptors (TLRs). TLRs are expressed on the cell surface and within intracellular vesicles (endosomes). TLRs are stimulated by the presence of pathogen-associated molecular patterns (PAMPs) such as cell wall/membrane components (eg lipopolysaccharide [LPS], peptidoglycan, flagelin) or intracellular components (eg single or double stranded RNA or DNA)6 . In general, each TLR has a specific “toll” required for stimulation, and more than one TLR can be stimulated simultaneously allowing for concerted responses to be produced10. Following TLR stimulation, second messenger-specific intracellular signaling cascades are activated that result in gene expression, cytokine/chemokine production and cellular activation6 .
A second important piece of immunology background relates to the effects of the cytokines produced following innate immune stimulation. Patterns of cytokine production are important because the cytokine milieu can promote the differentiation of naïve CD4+ T cells into distinct subtypes of TH cells that serve important roles in the clearance of pathogens9 . For example, TH1 cells are produced from naïve CD4+ T cells following exposure to interferon (IFN)-γ and IL-12, and support cell-mediated immunity against intracellular pathogens through production of IFN-γ, tumor necrosis factor (TNF), and lymphotoxin. TH2 cells arise in the presence of IL-2 and IL-4, produce IL-4, IL-5, IL-13, down-regulate TH1 responses, and support humoral immunity as well as defense against extracellular parasites. A third subset of TH cells, TH17 cells, are produced in the presence of transforming growth factor (TGF)-β, IL-6, IL-21, IL-23, produce IL-17, IL-22, and are important for defense against extracellular bacteria and fungi.
In subsequent sections, we will frequently refer to the cytokine milieu as TH1, TH2, or TH17-polarizing or promoting based on these descriptions. Though there are multiple cells that comprise the innate immune response, the neutrophil (also known as polymorphonuclear leukocyte) and antigen presenting cells (APCs; monocyte, macrophage, and dendritic cells) are each important to the neonatal response to infection (Figure 1)2, 4, 11, 12. Neutrophils have been examined in adult and neonatal infection as one of the primary responders to pathogen-induced inflammation11, 13, 14. The neutrophil is not only able to phagocytose and clear bacteria but it is also is able to release anti-microbial proteins and peptides (APPs), such as lactoferrin (Lf) and bacterial/ permeability-increasing protein (BPI), upon activation at infected sites15. The role of the macrophage is similar to the neutrophil in that it functions to clear dead or dying neutrophils and bacteria through phagocytosis but also plays an important role in shaping the adaptive immune response to pathogens, indirectly through cytokine secretion or directly through antigen presentation in secondary lymphoid organs such as lymph nodes and spleen1 . The dendritic cell (DC) also plays an important role in the innate immune response to pathogens and is critical in formation of antigen-specific immune responses (e.g. antibody production) and memory responses in both T and B cells16, 17 .
<h2>Why is neonatal innate immunity important to the practicing clinician?</h2>
The innate immune response is critical at every stage of human development because it regulates tolerance to self, generates vaccine or memory responses through the interaction with T and B cells, and provides early non-antigen specific pathogen protection to prevent infection. In most cases, defects in post-TLR stimulation second-messenger signaling intermediates critical to effective innate immune function, such as IL-1 receptor activating kinase-4 (IRAK-4) or myeloid differentiation factor 88 (MyD88)-deficiency28, will present early in life, either immediately following birth or within the first few months of life. This early susceptibility to infection is also readily observed in infants with diseases of innate immune system function such as chronic granulomatous disease (CGD) or leukocyte adhesion deficiency (LAD) that typically present early after birth with systemic infection or with persistent mucosal and respiratory infections with encapsulated bacteria throughout infancy. However, even in lieu of congenital innate immune defects, the developing neonatal and infant immune system is distinct at baseline with impaired generation of inflammatory responses to prevent infection.
Full reseach paper download now from GoogleDrive
Source
Plagiarism is the copying & pasting of others work without giving credit to the original author or artist. Plagiarized posts are considered spam.
Spam is discouraged by the community, and may result in action from the cheetah bot.
More information and tips on sharing content.
If you believe this comment is in error, please contact us in #disputes on Discord
this is a creative common article why you downvote me i mentioned author name and url?????
i have a full access to share it because it is creative common
Hi! I am a robot. I just upvoted you! I found similar content that readers might be interested in:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3959733/