(
What are Biosimilars?
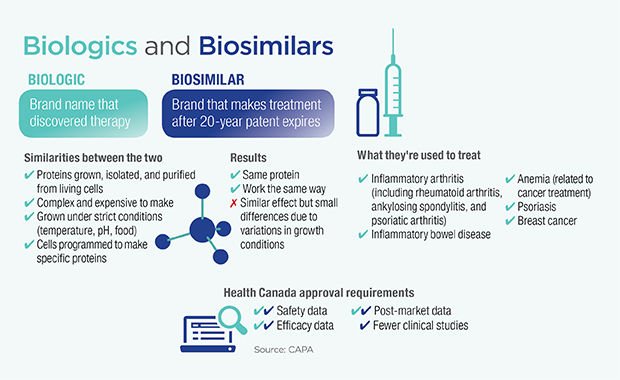
A biosimilar is a biologic medical product that is almost an identical copy of an original product that is manufactured by a different company.
Biosimilars are officially approved versions of original “innovator” products and can be manufactured when the original product’s patent expires. Reference to the innovator product is an integral component of the approval.
Difference between biosimilars and generics:
Biosimilars involve developing equivalent of biological entity while generics involve developing equivalent of a chemical entity-the Active Pharmaceutical Ingredient.
In case of biosimilars, biological entities being some ward different (and not as it is of replica), every organism has to be engineered to produce the same therapeutic effect while in generics, the copies of API can be generated
Bio-similars differ from generics – in complexity, in the manufacturing processes and in the data needed to demonstrate similarity for approval. The structure of Generic Simple and well-defined whereas for Bio-similar its Complex with potential structural variations.
Regulatory procedure to get approval for biosimilars is complex as compared to that of a generic.
Need of Biosimilars in India:
Due to enormous soaring demand for generic drugs, India’s pharmaceutical producers emerged as world market leaders in this sector and were a major business success story in the 2000s.
In the process, Indian producers made a valuable contribution to reducing costs and to expanding access to life-saving treatments for patients, both in emerging markets and in developed countries.
Recently there is a wave of consolidation among pharma retailers, stiffer competition from Chinese pharma manufacturers and an uptick in generic drug applications have combined to put downward pressure on drug prices.
It is imperative that India’s pharmaceutical manufacturers create new markets to restore market confidence in their growth prospects.
Fortunately, some positive moves in this direction:
There is a new push to produce more so-called complex generics.
These are hybrid medicines that often contain complex active pharmaceutical ingredients (the part of the drug that produces its effects) or formulations, or routes of delivery.
Things are still at an early stage in this segment but the signs are promising, with Indian firms having succeeded in capturing 19% of the global market in complex generics thus far.
Another, they would be well-advised to pursue is to expand their footprint in the biosimilars market.
Biosimilars are the generic versions of biologics medicines made from animal or plant proteins as opposed to chemicals.
Biologics are notable for targeting the underlying causes of diseases as opposed to just the symptoms, with fewer side effects.
Biologics are important market disrupters because they are transforming how we treat diseases, including certain types of cancer, rheumatoid arthritis, and multiple sclerosis.
The growth in the biosimilars market is welcome from a human development standpoint because they are more affordable than biologics, the high cost of which often puts them out of reach of many patients.
While it is encouraging to see Indian firms beginning to ramp up biosimilars production, there is a lot of room for additional growth. Biosimilars currently account for just $5 billion of the $240 billion global market in biologics.
Biosimilars: Targeted towards Non-communicable diseases (cancer, asthma, and arthritis):
There is an alarming spike across developing countries in the prevalence of non-communicable diseases.
To take one example, diabetes is fast becoming an epidemic in developing countries, with rates rapidly catching up with those of the developed world.
In India also, with 69 million diabetics in 2015, a number projected to exceed 100 million by 2030, according to the World Health Organization.
The number of diabetics across the South-East Asia region, which includes Bangladesh, India, and Indonesia, rose more than fivefold between 1980 and 2014, WHO has reported.
Therefore, promoting the production of complex generics and biosimilars can have a positive development impact given how targeted they are toward treating non-communicable diseases such as cancer, asthma, and arthritis.
Conclusion:
Therefore, we can see that biosimilars industry can act as a springboard for the pharma cos to innovate, excel and earn profit and the same needs to promoted at the earliest.
we need to increase access through affordable pricing and some of the drugs need to be under price control.
It is increasingly clear that the segment of the pharmaceutical market where we will see demand grow the fastest in the coming years is products that treat non-communicable diseases.
We should, therefore, strive to promote strong, indigenous producers of complex generics and biosimilars as this has enormous potential to improve public health in emerging markets.
Governments can support growth in production of complex generics and biosimilars by clarifying the regulatory framework for them, which is still evolving in many countries.
However, at the same time, a regulatory mechanism needs to be put in place and appropriate monitoring needs to be done to ensure that unfair and unethical practices are abstained from in preparation of biosimilars.
Hi! I am a robot. I just upvoted you! I found similar content that readers might be interested in:
https://www.medicalbuyer.co.in/the-need-for-growth-in-indian-biosimilars/