For the first time, researchers have been able to directly observe an atomic electron orbit and capture its wave action image using a new quantum monitoring device. And this remarkable device has given scientists the opportunity to continuously focus on the quantum field.
An atomic electron occupies space and creates orbit. But when scientists describe the finely tuned state of these substances, they have to rely on wave action. It is the mathematical method of describing the particle phase of a particle, i.e. the method of determining the uncertainty between its space and time. Quantum physicists generally use formulas such as Schrödinger's equation to describe this condition, which is expressed in many complex numbers and imaginary graphs.
Until reaching this state, scientists were not able to observe the actual state of the wave action. Trying to find the exact position of the atom or the unique position of the electron's velocity is very much like trying to catch a fish in one hand. On direct observation, quantum associations are in danger of being catastrophic. This is because of the need for a device that can measure statistical averages over time to capture the full state of the quantum.
But how does the propagation of a quantum particle expand or expand? The work was carried out using a quantum monitoring device as described by a group of international researchers. This device is directly visible to the molecular structure through photoionization. Aneta Stodolna, a researcher at the FOM Institute for Atomic and Molecular Physics in the Netherlands, described the physical review letter as he and his team plotted a hydrogen atom in the field of fixed (DC) electronic fields.
2
After hitting the molecule with laser rays, the tiny electron atoms rotate in the orbit shown by a special two-dimensional collector (a type of dual microscope placed vertically). There are several orbitals that you can use to reach the specified location of the electron collector. Researchers use this method to protect the wave action by disrupting its movement. To accomplish all of this work, researchers have used an electrostatic lens, which allows the outgoing electron wave to be twenty thousand times larger.
2
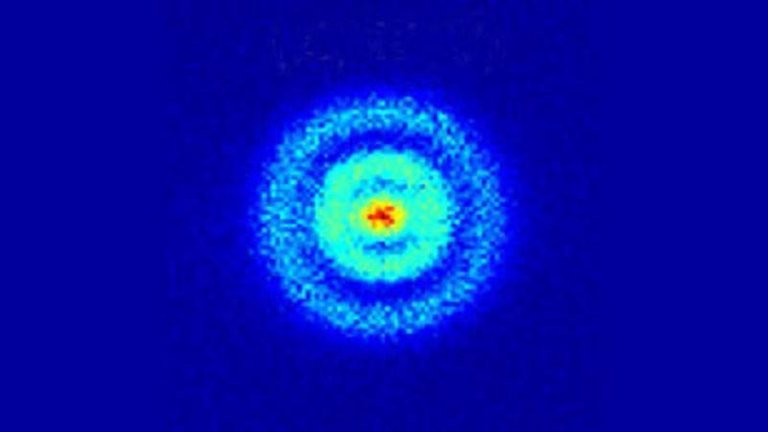
An example of four tens of hydrogen atoms is shown in the picture. The middle column shows the experimental measurements, where the right-hand column shows the time-dependent computation equation through Schrödinger and it fits neatly.
As a next step, researchers are using this technology to observe how an atom behaves in a magnetic field.

Congratulations @sahariar755! You have completed the following achievement on the Steem blockchain and have been rewarded with new badge(s) :
You can view your badges on your Steem Board and compare to others on the Steem Ranking
If you no longer want to receive notifications, reply to this comment with the word
STOPTo support your work, I also upvoted your post!
Vote for @Steemitboard as a witness to get one more award and increased upvotes!