Hi folks!
Today I have decided to have a series of thermodynamics review problem wherein I'm planning to make it daily or a day after. So today, the review problem is about the well-known law in the field of science of which thermodynamics is part of and that law is the Law of Conservation of Energy.
LAW OF CONSERVATION OF ENERGY
The law of conservation of energy states that
energy is neither created nor destroyed. And this where the first law of thermodynamics comes in, wherein it states that one form of energy maybe converted into another. So in other words, the energy that is being supplied to a certain thermodynamics will released or convert it to another form energy and it didn't mention that there is a decrease. So make it more understandable, the energy that enters a certain thermodynamic systems must always equal to the total energy that exits or leaves the system, which can be shown in the general equation below.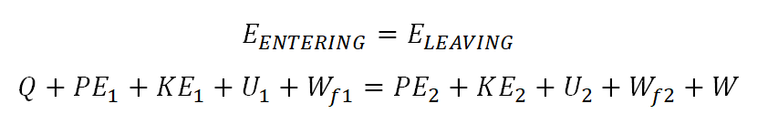
Where:
Qstands forheatPEstands forpotential energyKEstands forkinetic energyUstands forinternal energy- Wf stands for
flow work Wstands forworkdeltadenoteschange
To further learn about the the law of conservation of energy which is being applied in the first law of thermodynamics, let us solve a review problem. The review problem is stated below:
A thermodynamic steady flow system receives
4.56 kilograms per minuteof a fluid where p1 =137.90 kPa, V1 =0.0388 cubic meters per kilogram, v1 =122 meters per second, and U1 =17.16 kiloJoules per kilogram. The fluid leaves the system at a boundary where p2 =551.60 kPa, V2 =0.193 cubic meters per kilogram, v2 =183 meters per secondand U2 =52.80 kiloJoule per kilogram. During passage through the system the fluid receives3,000 Joules per second. Determine thework.
In the above review problem, it appears that we are being provided with the necessary parameters except for the elevation or height which is important in obtaining for the gravitational potential energy.
With that the first thing to do is to either create an illustration of the system or just taking into account the general formula for the law of conservation of energy. Actually to those a bit lazy students who usually don’t create drawings especially if it can be understood and I am one of those, the key here is to know the concept and calculations will just follow. As for this time, I choose not to create an illustration and just use the general equation.
Based on review problem, we have an equation for the energy balance of the thermodynamic steady flow system:
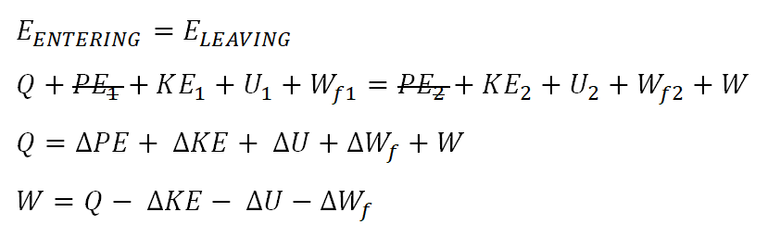
Notice that the equation doesn’t take into account the change in potential energy and that is because it wasn’t provided in the first place.
In order for the computation to be straight forward, all the parameters needed for the computation of
work will be solved and presented first so that by the time all these parameters are obtained, these can be directly substituted unto the formula which is shown in previous photo.So let’s start for the heat of the system, which is expressed in
Joules per second and reading the problem again, the word receives justifies that this one is of positive heat, meaning received by the system. Inspecting the given parameters we’d be able to notice that the mass is expressed in kilogram per minute and also other parameters are expressed in thousands with k such as kiloJoule (kJ) and kiloPascal (kPa) so with that we can either use kiloJoule per second or kiloJoule per minute; as for this review problem, I’ll choose kiloJoule per minute. And the simplification goes like this: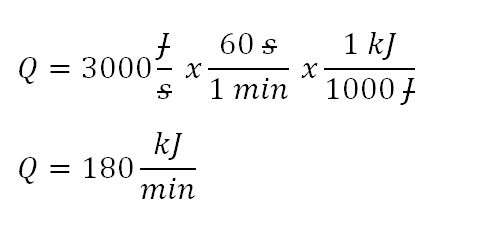
The heat that is being received by the system is equivalent to 180 kiloJoule per minute.
Next parameter to obtain is the change in kinetic energy that is being experienced by the fluid all throughout the system. And the computation goes like this:
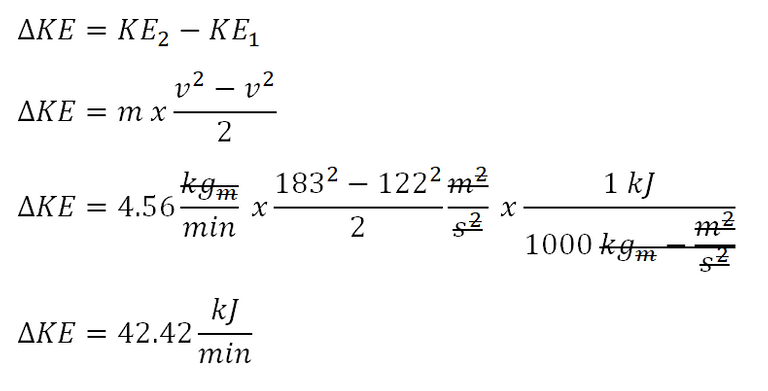
The change in kinetic energy being experienced by the fluid is equal to 42.42 kiloJoules per minute.
Next parameter to obtain is the change in internal energy wherein the computation is shown in the photo below.
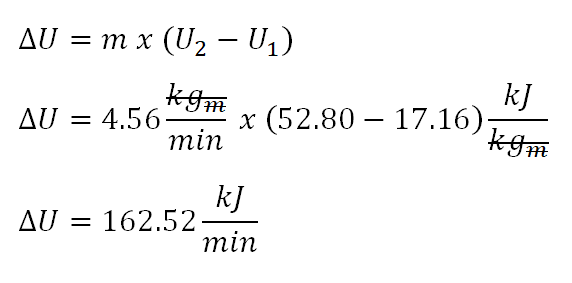
The change in internal energy being produced by the fluid is equal to 162.52 kiloJoule per minute.
The last parameter to be obtained is the change in the flow work being exhibited by the fluid from entering the system unto leaving the system. And the computation goes like this:
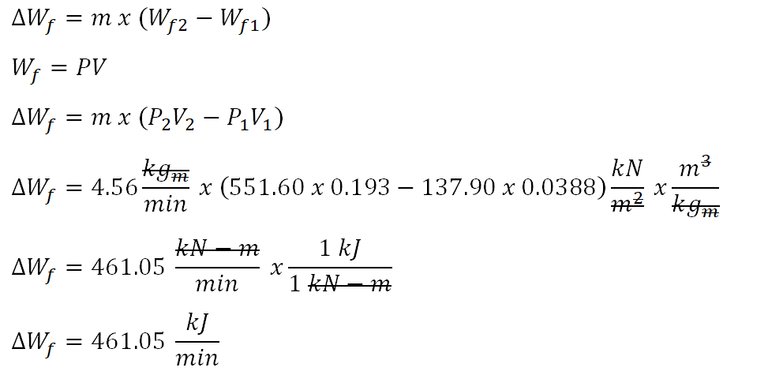
The change in flow work being exhibited by the fluid all throughout the system is equal to 461.05 kiloJoules per minute.
And since we have obtain all the necessary parameters that are important in the computation for the work of the thermodynamic system, we can directly substitute to the simplified formula suitable for this scenario. And the computation goes like this:
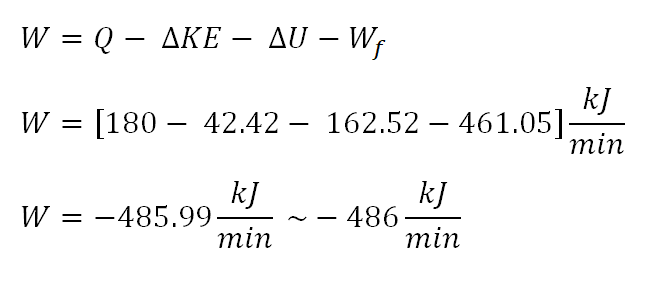
The work of the thermodynamic system is equal to -486 kiloJoule per minute, the negative sign indicates that the work is being done by the thermodynamic system to the surroundings.
Well, that's it for today's review problem, I hope you learnt something from this article. When I was still a student I'm a bit angry whenever I met problems like this one but as time passed by this has become easier and became just a basic especially when you reached some higher subjects/topics in thermodynamics like power cycles.
Thank you for spending your time reading this blog.
Much love and respect.
Ace | @josephace135
Reference:
Hipolito B. Sta. Maria, Thermodynamics
Solutions are being made by me and the sample review problem is found in page 34, problem number 4.
Presentation of equations and formulas were made possible by MS Word 2010 Equations function.
Screenshots were made possible by utilization of Snipping Tool application.
You have been upvoted by the @sndbox-alpha! Our curation team is currently formed by @jeffbernst, @bitrocker2020, @jrswab & @teachblogger . We are seeking posts of the highest quality and we deem your endeavour as one of them. If you want to get to know more, feel free to check our blog.