The division of matter.
Until 1827 it was assumed that matter could be divided as much as one wanted while the appropriate instrument was available. It is similar to building a clay figure: you can remove or add to your figure the amount of clay you want, which would be equivalent to a continuous representation of matter. Currently it is proven that there is a limit to this division, that is, the matter is discrete. Thus a discontinuous vision of matter is equivalent to building a figure with pieces of a construction game: you can also add or remove as many as you want but never less than one piece. The British John Dalton assumed that these pieces, the atoms, could not be divided even when they had a knife suitable for it. The continuity of the matter would imply being able to divide it indefinitely without ever reaching a minimum unit.
The Thomson model.
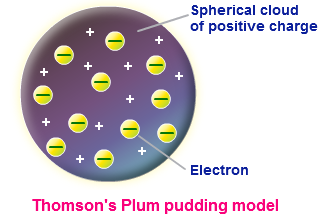
In 1898, Joseph Thomson produced cathode rays that he deflected by magnetic and electric fields. After several tests he concluded that they were negatively charged particles. Since the relation between its charge and its mass remained constant even when the gas inside the tube changed, it assumed that they were not atoms of the enclosed gas that were charged upon contact with the electrodes. If this were the case, the mass / charge ratios would be different depending on the type of gas contained in the tube. He guessed that the high voltage applied ripped the atoms inside the tube fragmented together. Dalton's indivisible atom had been split, it was no longer the smallest unit of matter. Thomson called these fragments electrons, which became the smallest possible piece of matter. In 1904 Thomson called these fragments electrons, which became the smallest piece of matter. In 1904 Thomson modified Dalton's atom model by imagining an atom model similar to a Plum-cake (raisins in panettone), consisting of a positive mass where the negative electrodes are embedded.
The Rutherford model.
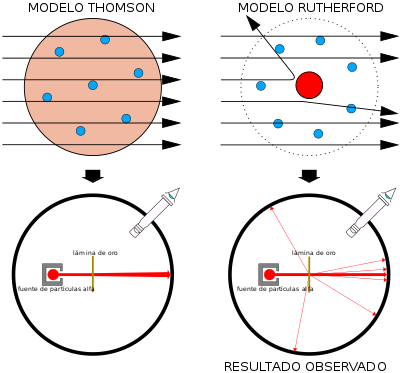
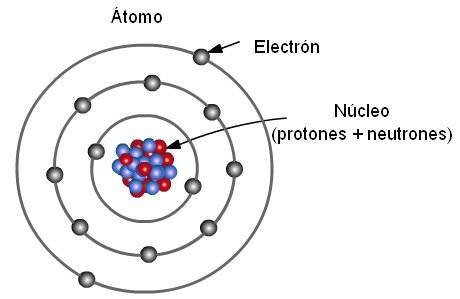
In 1911, the British physicist Ernest Rutherford, after bombarding with a narrow beam of particles a thin sheet of gold, found that most of the particles either did not deviate or did so at a very small angle. But, to his great surprise, he also observed that a few bounced off showing that a great repulsive electric force was diverting them. Rutherford supposed that this repulsion was exerted by a small region of positive charge that encompasses almost the entirety of the atomic mass, which he called the nucleus, and that its size was proportional to the number of particles bounced off. He concluded that the nucleus is made up of protons and neutrons. The remaining particles did not deviate, which showed that they found no obstacles in their path, which suggested that the rest of the atom was rather hollow.

The model of discord.
When scientists argue about a scientific theory, they do so based on the results of experiments performed. If these experiments have been carried out with rigor, the reason is granted to the researcher who has contributed them. This strategy was followed by Rutherford to defend his atom model. But this same reasoning was used by other researchers who recalled that hundreds of experiments showed that when an electric charge turns, it must emit energy. For the first time in the history of science there were experiments in favor of and finding of the same scientific theory.
The Bohr model.
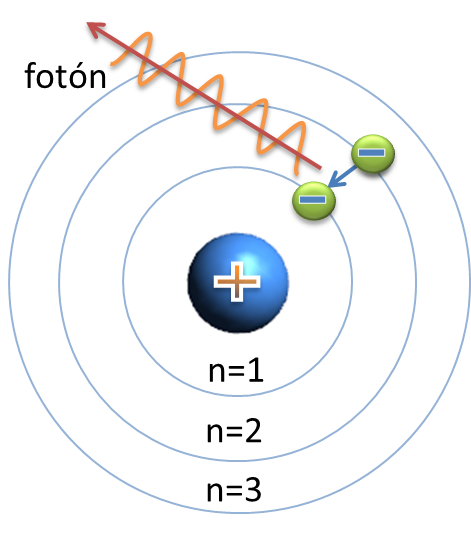
The new quantized atom model devised by Niels Bohr solved the contradictions between the Rutherford atom model and some phenomena discovered at the beginning of the 20th century: the photoelectric effect and the atomic spectra. By means of this model, the simplest spectrum, hydrogen, was justified, and the Ryberg constant was theoretically calculated, a fact that validated its acceptance. The new model of the atom involved recognizing an experimental evidence: it is not licit to suppose that the laws of macroscopic physics are valid for the world of the atom, since they may not be applicable. To fit in a single explanation all the known data, it was necessary to enunciate four new postulates and accept that the nascent atomic physics contradicted certain classical ideas about the behavior of bodies and commit some arbitrariness, as suppose the value of h / 2π.
The dimensions of the atom.
Imagine that you alternately place and lift two of your fingers inside a house located in an imaginary flat world. Your possible inhabitant would only detect the outline of the fingers, which would consider two different beings. I could not understand how they appear and disappear that are the only being the other world of three dimensions. It is impossible for us to imagine its real spectrum. We are in the same situation of the flat being of the previous example. We can only perceive the "contour of his footprint" in our three dimensional world. The components of the atom, when they are part of it, show a behavior that almost does not resemble that of a particle, that of a piece of matter. However, it is easily justified and understandable if one admits that electrons and other particles are not sufficient to justify all the characteristics of the components of the atom. In certain cases the behavior of electrons and other subatomic particles is better understood supposing that they are waves, while in others it is easier to justify what is observed considering that they are particles.
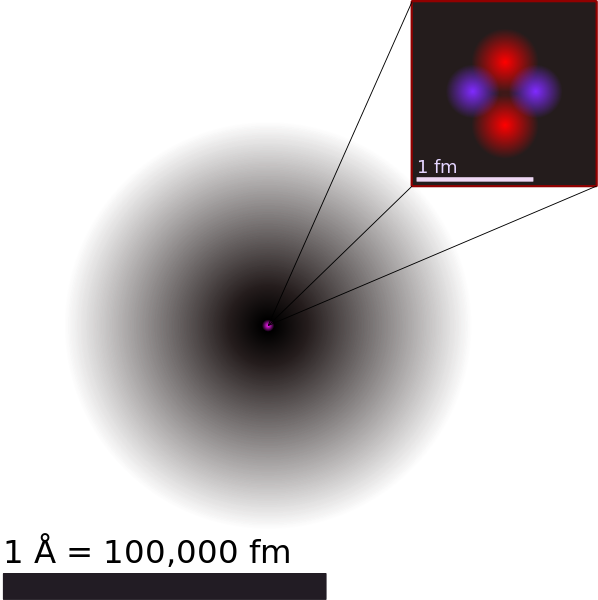
The bark of the atom.
An orbit is a representation of the behavior or characteristics of a particular electron. It is very important to note that an orbital is not a kind of shelf where the electrons are placed. If there is no such electron, there is no representation, that is, there is no orbital. In many practical cases the language used can lead to thinking like that. Care must be taken to avoid thinking of orbitals as shelves between which the electron jumps when losing or receiving energy. The situation is comparable to that of the tight rope and linked by its two ends to which different frequencies of vibration are applied. In the same way, the electron only exists for specific and allowed values of energy. For other different values, the electron disappears when not having the energy that is allowed.
Too bad you used images protected by copyright..
:,(
WARNING - The message you received from @tauwil is a CONFIRMED SCAM!
DO NOT FOLLOW any instruction and DO NOT CLICK on any link in the comment!
For more information, read this post: https://steemit.com/steemit/@arcange/virus-infection-threat-reported-searchingmagnified-dot-com
Please consider to upvote this warning or to vote for my witness if you find my work to protect you and the platform valuable. Your support is really appreciated!