Introduction
 (License: CC0]:
Pixabay
(License: CC0]:
Pixabay I guess it time to let the cat out of the bag and tell y’all my unhealthy candy secret as a child. I was having a conversation with my older cousin (two years older than I was at that time - I was 5 and she was 7), we were both enjoying our lollipops and appreciating every bit of it, even sending blessings and goodwill to the manufacturers, asking God to give them the strength to manufacture more :). In this process, a thought came to mind “How is this lollipop we love so much manufactured?” I immediately asked her and she replied “It’s made from sugar, it’s sweet like sugar so it’s definitely sugar” I popped another question immediately “if it’s made from sugar why doesn’t it melt so quickly in the mouth the way sugar does” she say quiet for a while and finally said something “It’s made up of candle and sugar that’s why it doesn’t melt the way pure sugar melts in our mouths”; this made so much sense to me at that time and marked the beginning of my first (attempted) home made candy :).
We ran out of candy and needed more so we got our kits ready to make our own candy. We got small measuring cups (the smallest size), matches, candles and granulated sugar. Here’s our trick, we poured the sugar in the cups and poured the melted candle wax into the cups and stirred immediately because you know candle wax solidifies almost immediately outside heat. We would then get the solidified candle wax mixed with sugar out of the cup and put in our mouths; sucking on it until it didn’t taste so good anymore. This was a failure for us because it didn’t come out the way we thought it would and we definitely couldn’t let it melt in our mouths the way lollipop did but still quenched our thirst for it for that time being :). I could almost hear you screaming this is really an unhealthy thing to do; I know right but what’s the fun in being kids if you didn’t do unhealthy things that seemed healthy and fun for you.
What’s the science behind making candy ?
 (License: CC0]:
Pixabay
(License: CC0]:
Pixabay The candy I thought I made as a child was literally shit and as I grew I realized that making candy was more than just melting candle wax and sugar and letting it solidify. The science involved in making candy is more complex than I thought it would ever be but it’s all chemistry and guess what ! Chemistry is super fun.
Let’s get to the real business, as I said earlier the processes involved in making candy isn’t simple and it is also faced with chemistry concepts that are somewhat challenging. Who would have known that the candies I loved so much as a kid were not from candy land but from chemist who made something beautiful and sweet out of science (chemistry) using chemical reactions; this chemical reactions occurs in sugar molecules. For us to understand how candy is made we need to first understand the reaction that occurs in sugar molecules since it’s the major component used in making candy.

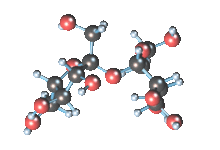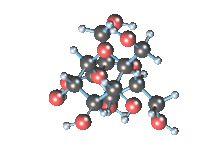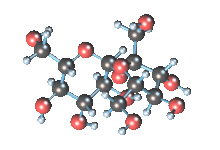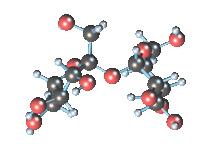
Wikicommons, Author: RedAndr CC BY-SA 3.0
The sugar molecule with chemical formula C12H22O11 has 12, 22 and 11 atoms of Carbon, Hydrogen and Oxygen respectively. The table sugar we know is called sucrose which is used in producing candy; it is a disaccharide with each molecule containing two monosaccharides which are glucose and fructose. Scientists take advantage of the sucrose structure to produce all kind of candies using barely sugar, liquid and perhaps fat. Someone may be wondering how cotton candy is produced using just these three items mentioned above; I’ll advice you to worry less because we will get to that part later in the article. The sucrose molecule loves keeping its shit together and could do anything just for it not to get split up but if it’s not split up how do we get our darling candies ? I apologize on behalf of candy lovers for always splitting you up, dear sucrose.
This splitting up is as a result of heating; when a sucrose molecule is heated up to the right temperature it melts down and turns brown in color with an accompanied nutty flavor, which is a process called caramelization. I love the sucrose molecule because it’s a fighter it doesn’t just let everything slide like ice when it melts to liquid. The sucrose molecule keeps fighting till the end (welll, when we suck on it until it’s no more :)), after melting it tries to stick together again by forming sugar crystals - Now this is the important part to take note in candy making as the level of crystals formed and the size of crystals formed would determine the nature of candy made whether glassy, hard-rock, fudge, geode and gritty (cotton candy) etc.
For us to make candy we need to first produce a supersaturated liquid. How is this liquid produced?
During the process of caramelization this liquid or solution is produced. This liquid or solution is gotten from heating sugar to a temperature where many sugar molecules are forced to melt and dissolve forming a solution which is thick and supersaturated. (This solution can’t be gotten by barely melting sugar in water because we would never get a caramel thick solution by just dissolving sugar in water.) The super saturated solution is what we use to make our dear candies; as I said earlier sugar molecules hate being split and would almost immediately start forming crystals to stick back together, so the rate at which the supersaturated liquid starts forming crystals and the size of crystals formed determines our final candy product. The process where sugar crystals are formed from the supersaturated solution is called crystallization and varieties of candies are made from this process. Candy can also be made from the process where no crystal is allowed to form from the super saturated solution.
Let’s see how the different forms of candy we consume are being achieved
Candies come in two forms; one which requires formation of crystals and one which do not require formation of crystals. The rock candy and fudge requires the formation of crystals as opposed to the cotton, glassy candies, marshmallows, lollipops etc which do not require formation of crystals.
Rock Candy (Large crystal formation)
 (License: CC0]:
Pixabay
(License: CC0]:
Pixabay
Now we understand the sucrose molecule in the sugar crystals but what happens when we dissolve our table sugar in water is interesting; the water molecule becomes really cheesy, it gets close to the sucrose molecule and binds with it, causing the poor sucrose molecule to be distracted until it’s stolen away from its crystal into the solution by the water molecule. That was a selfish and cheesy love story of one water and sucrose molecule; this can go on when they are more water/sucrose molecule but the solution becomes supersaturated when the sucrose molecule becomes more than what the water molecule can separate from its crystals.
We should note that the sucrose molecule are not static and as the water molecule try to pull some away from its crystals, other sucrose molecules are recrystallizing as the sand time. The solution becomes saturated when the amount of sucrose molecule that the water molecule separates from its crystals equals the amount of sucrose molecule recrystallizing. To make your candy rocky, I mean to make rock candy :) you have to add more sugar to a liquid than the liquid can dissolve at room temperature and you have to force more sugar molecules to dissolve by heating it up. We have spoken about the dissolving and heating up aspect of making rock candy; since the nature of candy depends on it’s cooling process, how does this supersaturated solution cool down to form rock candy ?
To make Rock candy out of this supersaturated solution, the cooling process is quite slow and may take up to 6 days to form. During cooling, the temperature of the solution obviously decreases but in an attempt to maintain equilibrium in the system, energy is generated by the system to increase the temperature of the system. Also in this process, chemical bonds are formed and when this bonds are formed energy is released causing the sucrose molecule to recrystallize quickly in a bid to increase energy. This crystallization increases as the temperature reduces which is how rock candy is formed because of the time period allowed for cooling and recrystallization of large crystals.
Fudge (Small crystal formation)
 (License: CC0]: Pixabay
(License: CC0]: Pixabay Fudge is a unique form of candy made also from saturated solution of sugar which has a unique smooth feel when we put it in our mouths. It is made up of smaller crystals when compared to rock candy which is one of the reasons for its smooth feel because you know well, the smaller the crystals the smother the candy. I said earlier in this article that the nature of candy you want to achieve depends on the cooling process; while rock candy is left to cool slowly without interrupting the cooling environment, making fudge is rather different. In order to prevent the saturated solution from forming large crystals, we are required to stir the saturated solution. This solution which must have been heated to a temperature of 116 C, known as the soft ball stage above the boiling point of water (100 C). When I said “stir the saturated solution” I meant stir the solution when it has cooled down to a certain temperature of about 43.33 degree Celsius undisturbed, because if the solution is disturbed during the cooling process or when it hasn’t reached the temperature its required to cool off to, and it’s disturbed, it causes the fudge to become grainy which would definitely not let us have that smooth fudge feel we desire when we put fudge in our mouths.
The stirring technique is quite reasonable, because when these cooled solution is disturbed continuously, a large amount or number of crystal is formed but in very small sizes. These small-sized crystal molecules are pushed against each other by the process of stirring causing formation of crystal seeds which increases as stirring continues and the crystals also becomes smaller as the crystal seeds increases. When making fudge, the syrup or saturated solution is required to cool a lot faster than the rock candy in order to limit formation of intermolecular interactions among sucrose molecules which aids in the formation of large crystals. As the crystal seeds forms successfully and the temperature decreases progressively, the sucrose molecules moves about and sticks to many seed crystals in a bid to make the crystals to remain relatively small which is responsible for the thick, smooth and sweet feel we have when we put fudge in our mouths.
Glass Candy, Lollipops, Cotten Candy (No Crystal Formation)
Unlike rock candy and fudge the main goal in making candies like glass candy, lollipops, Cotten candy, marshmallows the list could go on, is the ability to prevent crystallization during the production processes.
Glass Candy
 (License: CC0]: Pixabay
(License: CC0]: Pixabay
For the candy to successfully turn out to be a glass candy it has to cool off in a way that no crystal is allowed to form which causes the sucrose molecule to bind in an irregular pattern. The syrup is heated to 300 F which is referred to as the hard-crack stage before it’s cooling down process is accelerated in such a way that the candy turns out to be amorphous and appear glass like in nature. The glass candy has the fancy name - edible glass; quite fancy yeah ?.
Cotton Candy
 (License: CC0]:
Pixabay
(License: CC0]:
Pixabay
Lollipops
 (License: Public Domain]: Pixabay
(License: Public Domain]: Pixabay Conclusion
Candy science is really fun, looks simple but complex. Another interesting part of candy science is that we can use one sucrose syrup with sucrose as the major raw material to produce a variety of candy. This difference in type and nature of candy even with the single raw material is time period for cooling down and the activities carried out during the cooling process. Candy science to me, is actually sweet chemistry, as you toy with the size of crystals formed and even in some cases prevent the crystals from being formed in order to get your desired texture and form of candy.
The crystallization and recrystallization of candy is also illustrates the famous Le Chatelier's principle which states that
When any system at equilibrium for a long period of time is subjected to change in concentration, temperature, volume, or pressure, then the system readjusts itself to partly counteract the effect of the applied change and a new equilibrium is established. Source
I had fun writing this article, I hope you have fun reading it with candy treats.
References
Candy science
The science of making Candy
The sweet science
Sugar Science

Now that everyone knows the path to your heart, expect a cartonful of your beloved candies at your doorstep with an engagement ring from @agbona or @rharphelle.
The wax/sugar mixture adventure was a bit daring. You must have been one naughty little girl back then. Lovely article.
Thanks for pointing this our Mr Shaid, I'll work towards that 😁
And this is an amazing article @flora, seems like you had one hell of a childhood
I smiled reading this lol
Thank you Mr Shaid @gentleshaid
I was a little naughty and had wild cravings 😂😂 (I would say)
Thanks for stopping by @agbona
This post has been voted on by the steemstem curation team and voting trail.
There is more to SteemSTEM than just writing posts, check here for some more tips on being a community member. You can also join our discord here to get to know the rest of the community!
Congratulations @florae! You have completed the following achievement on Steemit and have been rewarded with new badge(s) :
Click on the badge to view your Board of Honor.
If you no longer want to receive notifications, reply to this comment with the word
STOPHi @florae!
Your post was upvoted by utopian.io in cooperation with steemstem - supporting knowledge, innovation and technological advancement on the Steem Blockchain.
Contribute to Open Source with utopian.io
Learn how to contribute on our website and join the new open source economy.
Want to chat? Join the Utopian Community on Discord https://discord.gg/h52nFrV