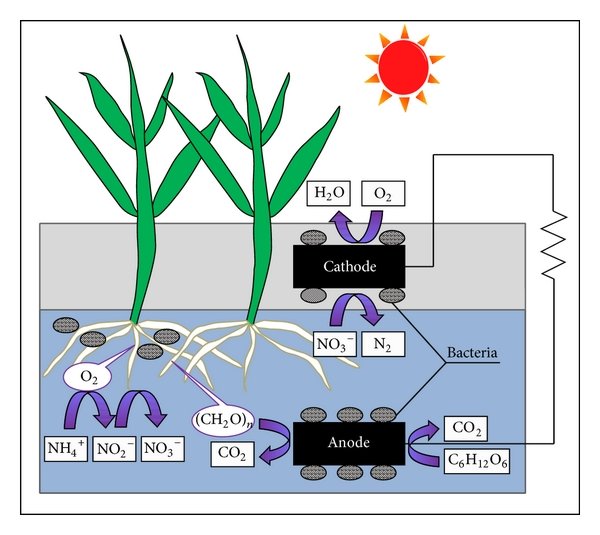
Besides energy from the sun and wind, a plant microbial fuel cell (PMFC) is a new way to produce electrical energy. This Plant Microbial Fuel Cell is a bio-electrochemical system where micro-organisms degrade organic matter and produce electrons. These electrons travel through enzymes in the cell and make energy for the cell. Through photosynthetic effects this PMFC is able to gain electric current from energy the plant gets from sunlight. This system is capable of supplying green energy 24 hours a day, seven days a week. Which makes the world wonder if this will be our number one energy source in the near future. The direct current which is produced in this manner has a low voltage (1V) and as such is not dangerous for animals or plants. This process occurs in 7 steps, as explained down below.
As discussed above, plants are able to gain electric current through photosynthesis. A plant produces organic substances and oxygen from carbon dioxide and water thanks to photosynthesis (step 1). Over 65 percent of these organic substances will be transported to the roots of the plant, which is the second step. Near the roots these organic substances are broken down by bacteria via an oxidation process. In this reaction, electrons, protons and carbon dioxide will be released. An example of a reaction during this process is shown in formula 1 where the oxidation reaction of glucose is shown.
𝐶6𝐻12𝑂6 → 24𝑒− + 6𝐶𝑂2 + 24𝐻+
In a PMFC, the released electrons will be transported to the anode by the bacteria (step 4). This anode is an electrode with a negative pole. Since the anode is defined as the electrode at which the oxidation reaction takes place. These electrons will be transported to the cathode, the positive pole, by an isolated wire. An electrical device can be connected between the anode and the cathode (step 5). The anode and the cathode are separated by a membrane which only lets protons (H+) through. This makes it possible for the protons to move from the anode side to the cathode side (step 6). The cathode is half submerged in water, the other half receives oxygen from the air. So the cathode receives protons, electrons and oxygen. These will be converted into water using formula 2 (step 7).
𝑂2+4𝐻++4𝑒−→2𝐻2𝑂
At the anode side in the PMFC, electrons are released via the oxidation reaction as discussed. These electrons are positioned in the water around the anode, but they have to be transferred to the anode. In an MFC, chemicals are often used to transfer the electrons. These chemicals that can transfer electrons are also called mediators.
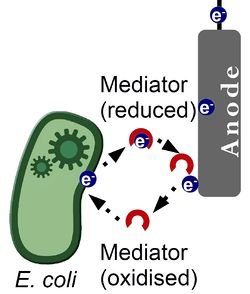
The PMFC does not use a mediator and therefore it is called a ‘Mediator-less MFC’. It is not fully known how the electrons are transferred to the anode in these cells. Two processes that influence the electron transfer to the anode will be discussed in this article, but there might be more factors which are not discovered yet.
Direct electron transfer
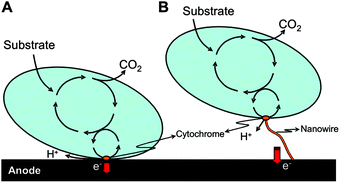
During direct electron transfer, electrons are transported via physical contact between the cell membrane of the bacteria or the membrane organelle and the negative electrode. No reactions will occur during the transmittance of the electron. First this process was considered as impossible, since living cells were considered as non-conducting, but for some cells it is possible to transfer an electron from its center to the membrane. The efficiency of the electron transfer to the anode is depending on the amount of bacteria that can live close to the anode, since physical contact between the bacteria and the anode is required.
Nano Wires
For some bacteria it is possible to transfer electrons to the anode without being in physical contact with the anode. They use electrically conducting strains called ‘nanowires’. They are attached to the membrane-bound cytochromes. Because of these nano-wires, bacteria that are at a larger distance from the anode are still able to transfer electrons to the anode. This will cause a higher efficiency. In the figure down below, the direct transfer of electrons (A) and the transfer via nanowires (B) is schematically presented.
Internal resistance
The opposition to the flow of current within the PMFC is called the internal resistance. This internal resistance is influenced by two components; electronic resistance and ionic resistance. The sum of those two is the total effective resistance.
The electric resistance is caused by the resistivity of the materials the current flows through and by how well these materials make contact to each other. The ionic resistance is caused by the efficiency of the biochemical processes in the PMFC. For example this can be influenced by the material and size of the electrodes, the used bacteria or the plants.
When the electrode potential of a PMFC is measured. It is important to consider the internal resistance of the cell.
Even though our daily electricity consumption is much higher than can be handled by small renewable PMFC, it seems like the world is only a few experiments away from replacing the biggest energy source.
Would be nice if they can scale this energy source up.
Agreed! Researchers are still working on it though, so maybe in the near future? Guess we'll have to wait :/
Interesting reading. Thanks for sharing.
Thank you so much!
Congratulations @rocketscience! You have received a personal award!
Click on the badge to view your Board of Honor.
Do not miss the last post from @steemitboard!
Participate in the SteemitBoard World Cup Contest!
Collect World Cup badges and win free SBD
Support the Gold Sponsors of the contest: @good-karma and @lukestokes
Congratulations @rocketscience! You received a personal award!
You can view your badges on your Steem Board and compare to others on the Steem Ranking
Vote for @Steemitboard as a witness to get one more award and increased upvotes!