
The second lecture in this week was "PrEP: Pharmacodynamics / Pharmacokinetics" introduced by Neha Pandit, an assistant professor of practical pharmacology at the School of Pharmacy at the University of Maryland (USA).
The aim of the lesson was to explain the basics of the tenofovir and emtricitabine pharmacodynamics, as well as a mechanism to prevent the transmission of HIV. Also, the instructor will tell about the time to the protective concentration accumulation of the drug in mucosal tissues, sufficient to protect the body from HIV. Finally, the instructor will provide with information on the new medications and administration alternatives already in the approval pipeline for PrEP.
First, says Neha, let’s talk about the mechanism of action of tenofovir and emtricitabine — both of these molecules are a component of Truvada, approved by the FDA for HIV pre-exposure prophylaxis.
Tenofovir disoproxil fumarate, abbreviated as Tenofovir or TDF, belongs to the class of nucleoside reverse transcriptase inhibitors. Emtricitabine, FTC, belongs to the class of nucleotide reverse transcriptase inhibitors.
TDF and FTC start to work only in the active state. To transform into the active state, they would need to get into the cell. Once a patient takes medication in orally, it then gets absorbed into plasma and then gets converted or pushed into the HIV target cell, such as the lymphocyte, where both emtricitabine and tenofovir undergo a little transformation, named ’phosphorylation,’ using internal cell proteins. Emtricitabine gets converted to emtricitabine 5- triphosphate and tenofovir gets converted to tenofovir diphosphate. Once these medications turn into their active form, they get integrated or incorporated into the HIV DNA, terminating that HIV DNA chain. That’s how it prevents HIV replication from occurring.
Next, the instructor explained the ideal conditions necessary for pre-exposure prophylaxis.
Tolerance and safety
Let’s move back a little back and look at the late 90s and early 2000s. Most of the drugs that existed at that time had severe toxic effects to patients and actually should not have been used for a long period. Nowadays, much safer drugs becoming marketed, so that’s budding situation for PrEP comes to play.
Truvada has good tolerability and safety. Studies have shown that in combination, tenofovir and emtricitabine slightly affect the liver and kidneys, in rare cases cause weight loss, and can also cause nausea, vomiting, or diarrhea. Compared with other drugs, the frequency of such incidents of intolerance is much lower. People taking other drugs often complain of depression, hallucinations, skin irritations, local loss of sensitivity. Such adverse drug reactions sometimes encourage skipping pill intake and even stopping the course.
But the combination of TDF + FTC is devoid of such adverse reactions, and this is good news for PrEP.
Low pill burden
Let’s return to the USA. The emergence of new safe drugs that are well tolerated, without serious side effects, has become an ideal situation for discussing PrEP in the professional community. It was necessary to solve one more puzzle to enable the possibility for people without HIV of taking tablets to prevent HIV, — to place therapy in one tablet. After all, until the end of 2000s people had to have drink three or four tablets a day, and at the dawn of antiretroviral therapy, it was necessary to take the pills literally with handfuls several times a day. It would be unthinkable to offer the same form of taking toxic medications for people without HIV. Therefore, professional communities were expecting new breakthroughs from science.
And such a breakthrough happened when the Truvada, a combination of emtricitabine and tenofovir, was presented on the market. Studies have shown that for effective prevention of HIV transmission, only one tablet of Truvada per day is sufficient.
Half-life
How had scientists designed antiviral drugs in one tablet achieving high efficacy while taking the pill once a day? They were looking for a way to increase the half-life — the period of time required for the concentration or amount of drug in the body to be reduced by one-half. At the beginning of the lecture, Dr. Pandit said that tenofovir and emtricitabine act only inside the cell. The half-life of tenofovir is 60-150 hours, emtricitabine — 39 hours, thanks to the intracellular transformation of the drug. As we remember — Truvada should be taken once a day, so in the human body there is never happens a drop in concentration more than twice. The concentration of the drug is always 50% or higher since the body will need 39 hours to eliminate more than a half of the phosphorylated active tenofovir from the lymphocyte cells, and at least 60 hours to remove more than a half of the phosphorylated active emtricitabine.
So, PrEP started to be provided in one tablet, which could be taken once a day.
High barrier to resistance
Another essential demand for pre-exposure prophylaxis is a high impediment to the formation of drug resistance to the drug in the virus. The resistance for tenofovir and emtricitabine is relatively small for the most part. And so those are ideal characteristics for those medications. The study showed that Truvada worked efficiently in the body of macaques, resistant to emtricitabine.
High concentration in mucous membranes
And the last requirement is whether the drug reaches high levels in the mucosal tissues. Mucosal membranes in the mouth, vagina, and rectum are exposed to the other person’s biological fluids in case of being the receptive anal partner for sexual activity or being an inserted vaginal partner.
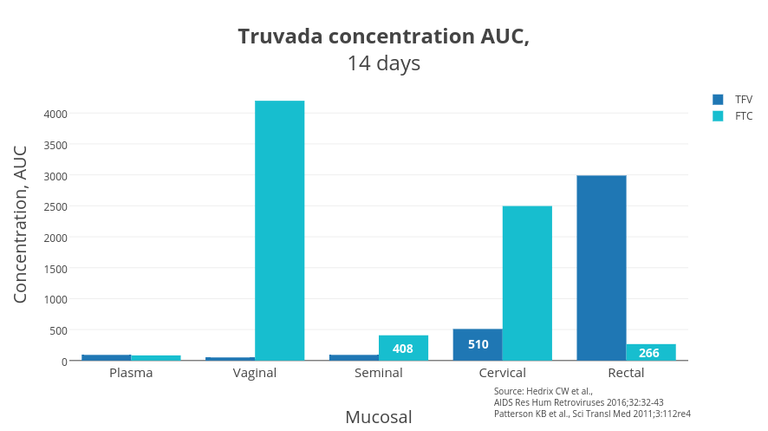
Figure 1 — AUC Truvada concentrations in mucosa tissues
The graph (Figure 1) shows the difference between the AUC-concentrations of tenofovir and emtricitabine in the tissues. The AUC concentration or area under the concentration-time curve (AUC) is the area of the figure bounded by the pharmacokinetic curve of the drug concentration in the tissue at each time point and the coordinate axes. Thus, the numbers that you see on the chart are the total amount of the drug that was in the tissue for 14 days. The more the drug was in the organ’ mucous tissue for 14 days — the more protected from HIV the organ was.
Although the plasma concentration was somewhat similar, the differences in concentrations in the vagina are significant. Emitrcitabine works better in the vagina and cervix in women and in seminal vesicles in men. Tenofovir works better in the rectum in humans of both genders. Now it is evident why the combination of two drugs is best suited for PrEP; they build a secure shield against HIV in the mucous membranes of all vulnerable tissues of the body.
In plasma, the drugs reach the required concentration after two days after initiating the course, in red blood cells (PBMC) — after seven days. Therefore, in theory, to achieve a sufficient concentration in the body, it is necessary to take the drug for at least seven days. In practice, it is advisable to start taking PrEP one month before dangerous contacts happen.
These days multiple investigational pre-exposure prophylaxis medications are being evaluated. Some of them are already approved antiretroviral drugs that are currently used for HIV treatment are being examined as PrEP like tenofovir alafenamide. There’s also new classes of medications are being evaluated for pre-exposure prophylaxis, that’s noteworthy of neutralizing antibodies.
Various methods of drug delivery into the body are being studied, such as:
- vaginal rings;
- anal inserts;
- rectal gel;
- vaginal gel;
- self-dissolving films;
- implants;
- long-acting injections;
- solutions for the rectal douches.
All things that are relatively early in their stages for evaluation for PrEP. Such techniques and devices can occasionally be used or implanted once a month, six months or a year. The main idea pursued by the authors is to allow a person to avoid the need for daily intake of tablets for PrEP.
The lesson is over today, and we are waiting for you tomorrow at talk “Emerging Data/Ongoing Trials for PrEP” lecture.
If you have not already signed up for the PrEParing course on the Coursera platform — it’s never too late. Just go to https://www.coursera.org/learn/prep.
P. S. The original article was posted on the Life4me+ mobile app for HIV-positive persons blog.