
Coloured glass has been a source of wonder for centuries. Even today, nothing can match the intensity of the colour experience of glass colours. This older post of mine is about the stained windows in my grandfather's house, which bring back memories from my childhood. Probably my first impressions of colored glass - and still very vivid !
This is a new post on how these colors are made; a small overview of these colours and what stuff they're made of.
Chemistry
Making coloured glass is like making a dish: there are countless recipes. Standard glass consists mostly of Silica, with some Calcium and Soda-oxide added (i.e. Soda-lime glass). Standard clear glass is often a bit greenish, which is caused by small amounts of iron oxide (already present in the main ingredients). The image below shows some of the best-known ingredients that can be added to this clear glass to give it colour.
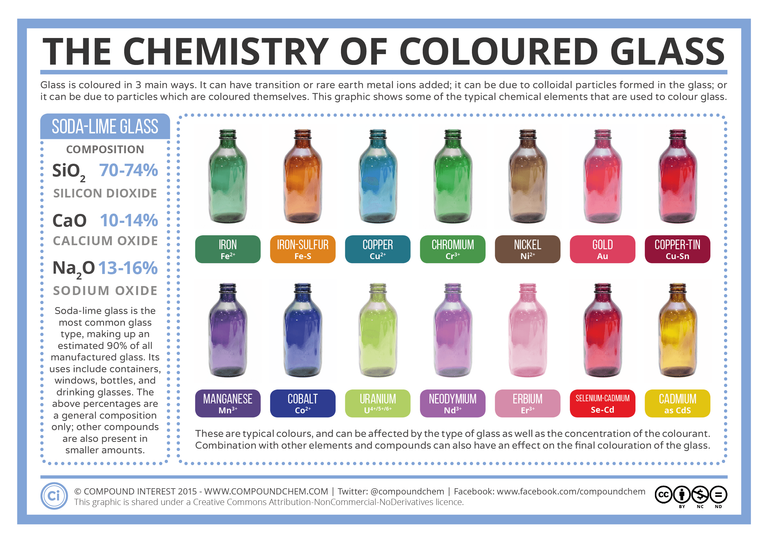
sources: here here and here. And here too as pdf
Sorted by color
The colors in the image below aren't very accurate, but it gives an idea about what metals can be used to obtain a desired color.
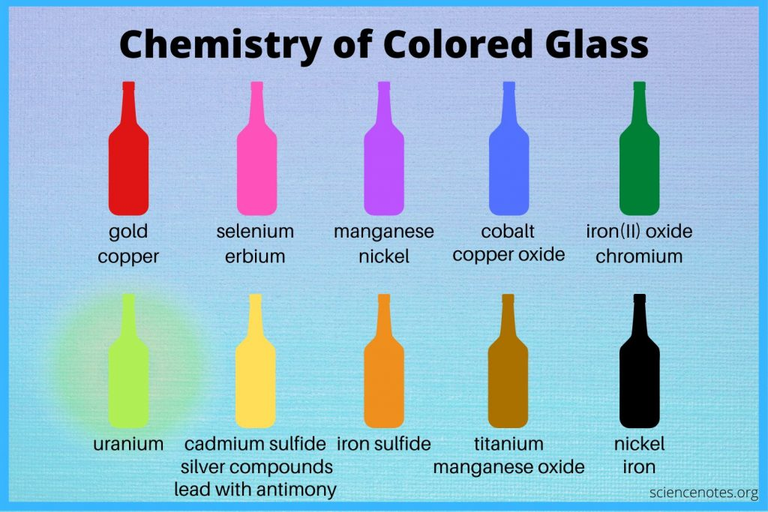
A non-exhaustive list of chemical compounds and the colour they give. This list is largely compiled from the sources listed at the bottom of this blog.
| Compound | Color |
|---|---|
| Antimony oxide | White |
| Cadmium sulfide | Yellow, Yellow-Orange fluorescence in UV |
| Cadmium Selenium sulfide | Yellow-Orange-Red, "Selenium Ruby" |
| Chromium(III) oxide | (Deep) Emerald Green |
| Chromium(VI) oxide | Light Green |
| Cobalt(II) oxide | Dark/Deep Blue |
| Metallic Copper (+ Tin) | Red |
| Copper(I) oxide | (Brown)Red |
| Copper(II) oxide | Blue-green, turquoise |
| Metallic Gold | Red, "Ruby Gold", used in "Amberina" |
| Gold chloride | Red |
| Iron(II) oxides | (Blueish)-green |
| Iron(III) oxides | Yellow-brown |
| Iron Sulfur | Orange Brown |
| Manganese oxide | Amethyst |
| Selenium | Pink (in high concentrations) |
| Sulfur oxides | Yellow or amber |
| Uranium oxide | Fluorescent yellow/green |
| Erbium(III) oxide | Pink, infrared absorbing |
| Neodymium(III) / Didymium (mixture of Praseodymium & Neodymium) | Pink-Purple |
| Praseodymium(III) | Yellow green |
| Metallic Nickel (?) | Blue, Violet, Black |
| Nickel(II) | Brown |
Yellow, Amber & Red
Yellow and amber glass, especially the vintage ‘Amberina’ glass, is made with Cadmium Sulphide (CdS), with gold for the red colour. This kind of glass will glow in UV-light with a yellow or orange colour. Also Copper

Amberina - Cadmium Sulfide + Gold
Copper red glass

Higher concentrations of CdSe and CdS produce a red glass color, sometimes called "Selenium Ruby". Copper(I)oxide also gives a red colour, albeit much darker and brownish.
Blue
The main colorants for obtaining blue glass are Cobalt (Co) en Copper (Cu) oxides. The latter gives a greenish blue or even turquoise, the first a deeper blue which might seem purple. Lower concentrations of cobalt can produce a colour easily mistaken for copper blue glass; see the vintage bottle in the picture below on the left.
 |  |  |
|---|
Old Pink - Selenium
Probably low(er) concentrations of Cadmium Selenide produces pink glass. This kind of pink glass may also glow pink in UV-light.


Other
It seems like people experimented with every new element as soon as it was discovered. Often successfully to create extraordinary and even fluorescent colours.


Congratulations @bartman67! You have completed the following achievement on the Hive blockchain And have been rewarded with New badge(s)
Your next target is to reach 700 upvotes.
You can view your badges on your board and compare yourself to others in the Ranking
If you no longer want to receive notifications, reply to this comment with the word
STOP