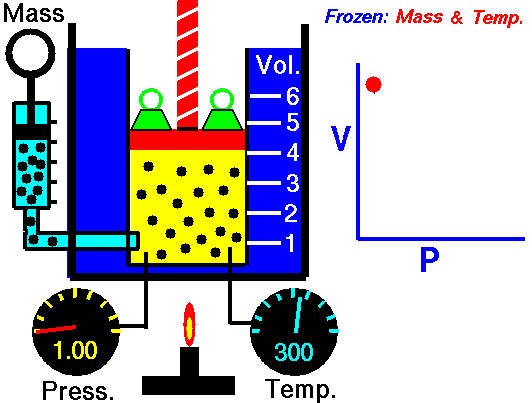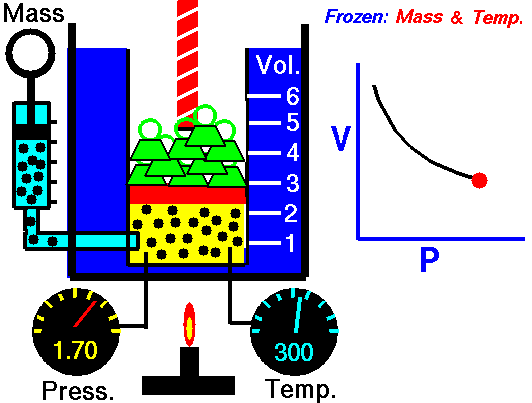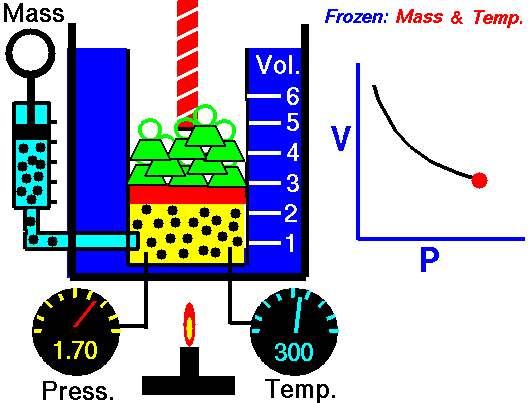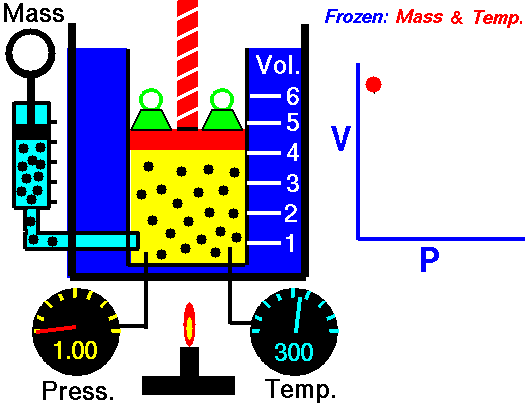
When we study physics it is very common to see a set of equations that follow the model to calculate work but when the force is constant, however when the force is already variable is necessary to resort to the knowledge of integral calculus, this is because the force that is applied to the object varies at the same time that also varies the position of the object, so we can define the calculation of work as follows:
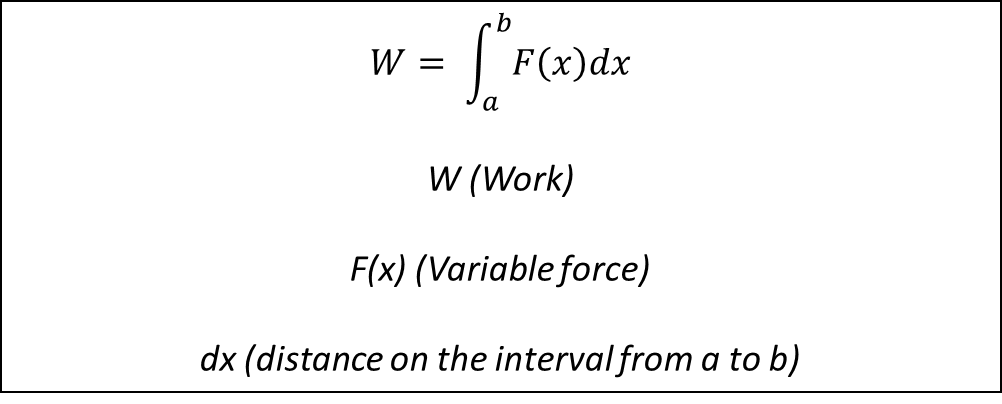
Definition of work done with a variable force
If an object is displaced along a straight line by a continuously variable force F(x), then the work W done by the force when the object is displaced from x= a to x= b is:
Application of the integral to calculate the work done for an expanding gas (Boyle's law for ideal gas)
If the volume of a gas expands from an initial volume V0 to a final volume V1, the work done (W) moving a piston is:
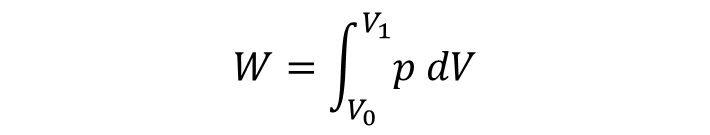
If we assume that the pressure (p) of the gas is inversely proportional to its volume, it follows that:
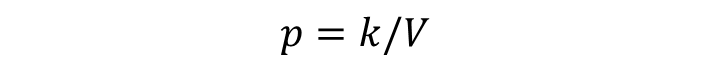
Therefore, the integral to calculate the work is:
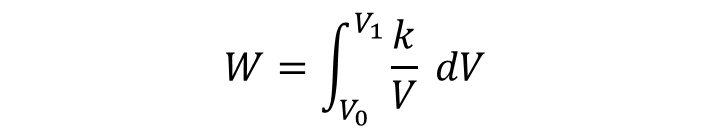
Exercise
Find the work done by a gas following Boyle's law, i.e. at constant temperature the pressure is inversely proportional to the volume, where its initial volume is 2 ft3 and its pressure is 1000 pounds per ft2 to a final volume of 3 ft.3
Solution:
Since P = k/V, it implies that
k = P V
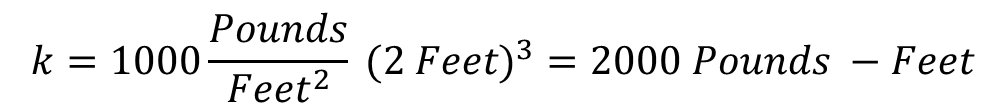
Therefore, the integral remains:
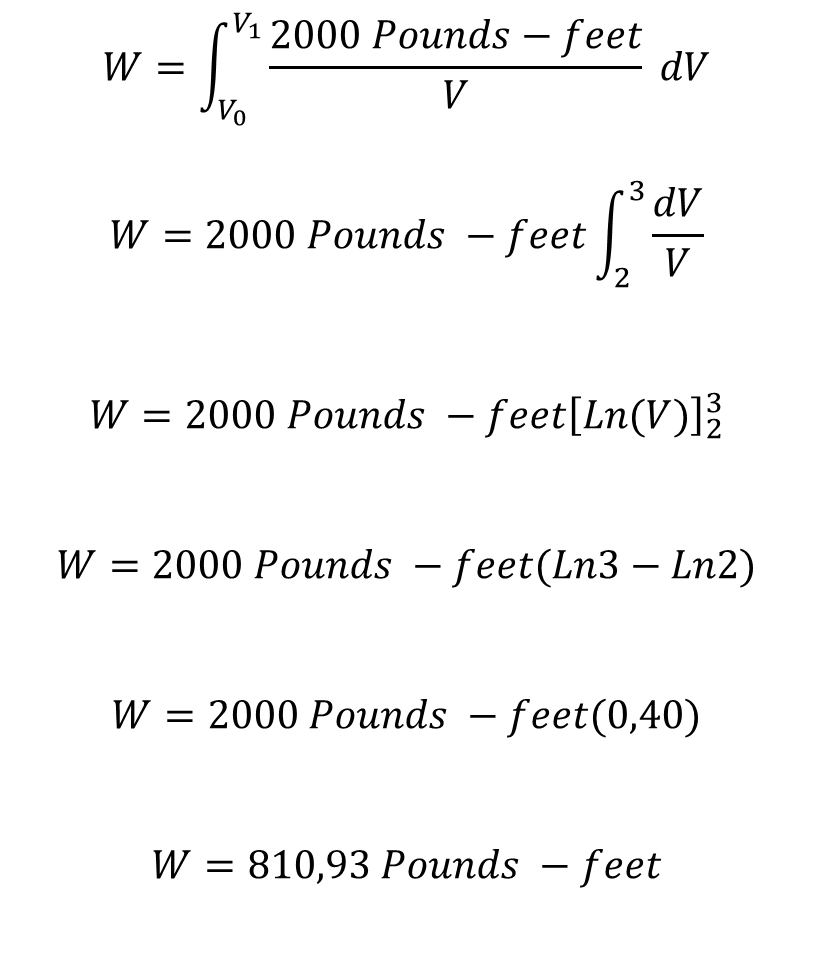
Conclusion and Analysis of the results
Following Boyle's law for a gas whose pressure is inversely proportional to the volume and that expands with a pressure of 1000 pounds per square foot and where its volume varies from 2 cubic feet to 3 cubic feet, it is obtained that it exerts a work W in its expansion of 810.93 pounds - foot, which is a considerable energy, whose power will depend on the time in the expansion process.
Another aspect to consider is that whenever we solve the integral it will be the natural logarithm of the volume, the calculation of the work will only depend on the limits of integration and the value of K.
Bibliographic Reference
Calculus with Analytic Geometry by Ron Larson, Robert, P. Hostetler and Bruce H. Edwards. Volume I. Eighth Edition. McGraw Hill. Año 2006
Wow! What an amazing lesson!! The animated image of the post cover is very clear
Hello my dear friend @stefano.massari. Certainly the cover image is very explicit, so anyone can clearly understand that the behavior of a gas at constant temperature makes the pressure inversely proportional to the volume, this means that if the volume decreases the pressure increases, and if the pressure decreases the volume increases, this is clearly seen in an embolo-piston system as shown in the figure.
The other important thing to highlight is how we can calculate the work generated by an expansion force of an ideal gas by means of integral calculus.
Greetings my dear friend and thanks for always supporting the interaction of my posts.