Ion exchangers are used very widely in the field of industrial water treatment. In recent years, following the development of polymers that are impervious to induction processes and that do not produce dangerous materials, ion exchangers have become widely utilized in the production of drinking water. It should be noted that while this approach was once forbidden for use in drinking water treatment, it is now most commonly used in drinking water softening as well as the elimination of some toxic metal ions. In industrial water treatment, the usage of ion exchange technology ranges from eliminating hardness and salinity to particular uses in removing certain ions.

Figure-1: Cation/anion ion exchange used in water purification of boiler feedwater.
- Ion exchange as a method of water softening:
Sodium-cycle water softeners are the most often employed ion exchange method for hardness removal, where the calcium and magnesium ions that cause hardness in the water are substituted by sodium ions.
Typically, the ion exchange softening apparatus consists of two columns which contain the ion exchanger. Additionally, it necessitates that the structural materials used to construct the exchange columns be both able to endure high pressures during operation and resistant to oxidation.
Ion exchanger manufacturers often provide comprehensive information about their products, including practical capacity, total capacity, renewal conditions, and additional details. Table 3 illustrates the total and usable capacities of several ionic exchangers:
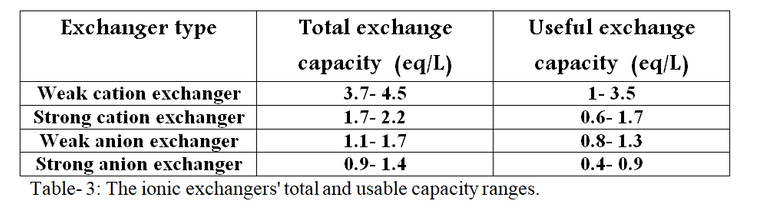
[Made using Microsoft Word]
According to the following regulations, the size of the column that contains the ion exchanger must be determined:
- The height of the area above the exchanger's surface should be approximately half as thick as the exchanger.
- The exchanger within the column must be higher than one meter in height.
- A gravel and sand filter layer with a thickness of at least 50% of the exchanger layer is positioned at the bottom of the column.
- Water moving through the exchanger layer should not have a surface velocity more than 18 m/h.
References:
- [Introduction to Water Chemistry (Pollution- Treatment- Analysis). Dr. Nasser Al-Hayek. Publication of the Higher Institute for Applied Sciences and Technology (HIAST). Syrian Arab Republic, 2017.]
- Taparhudee, Wara (2002). "Applications of Paddle Wheel Aerators and Diffused-Air System in Closed Cycle Shrimp Farm System" (PDF). Witthayasan Kasetsart (Sakha Witthayasat). 36: 408–419. Retrieved 26 April 2020.
- Unsafe water kills more people than war, Ban says on World Day". UN News. 22 March 2010. Retrieved 10 May 2018
- Raymond Desjardins- Livre: Le traitement des eaux- 2éme edition- Ecole Polytechnique de Montréal- 1997- ISBN 2-553-00643-8
- Drinking Water Treatment- EDX- Delft University of Technology.
- Book- Drinking Water: Principles and Practices- by Hans J C Van Dijk (Author), Jasper Q J C Verberk (Author), Peter J De Moel.
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
You may also include @stemsocial as a beneficiary of the rewards of this post to get a stronger support.