Have you ever wondered what fireworks are, when they were discovered, and what is needed to create their colors?
Of course, as with many things, the answer lies in chemistry…
The Origins of Fireworks
Gunpowder was one of the most famous discoveries of Chinese alchemists and significantly influenced the development of civilization. Some early recipes from the 4th century contained a mixture of potassium nitrate and sulfur. The first known complete recipe for gunpowder, or "fire chemical," which included charcoal as a third ingredient, was published in 1044 in the "Collection of the Most Important Military Techniques."
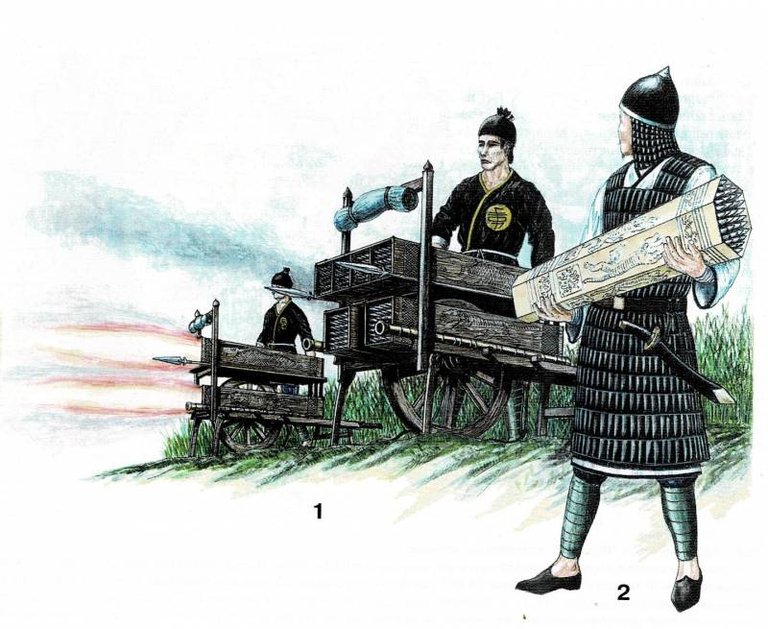
Picture taken from articlle "The most ancient firearm: where did it all begin ?!" Chinese missilemen of the Ming dynasty. Rocket launcher for shooting "fire arrows", 1450 (1); shooter with a rocket container (2). Drawing by a contemporary artist based on ancient Chinese miniatures
This demonstrates that the use of gunpowder quickly followed its discovery. By the 12th century, the Chinese were already using gunpowder for fireworks, primarily to mark important events such as births and deaths. Fireworks were also used for ritual purposes, believed to scare away evil spirits. Chinese New Year celebrations were almost always accompanied by fireworks, a tradition that continues to this day.
In the 13th century, in addition to military applications and trade caravans, Christian priests and monks played a significant role in the transfer of gunpowder knowledge to Europe (although the Arabs had their own gunpowder recipes by around 1270 AD). Over time, fireworks became a part of European culture, used to mark various events and celebrations.
My Own Experience with Fireworks
Growing up, I was always fascinated by fireworks. Colorfull explosions in the night sky felt almost magical. But what intrigued me the most was understanding how they worked. Curious as I am, I immediately went looking in books, why, what and how does it works. As a teenager, my curiosity led me to experiment with simple mixtures, always ensuring that safety was my top priority (deserted remote places where no one can get hurt, except maybe me, but that's another story). I remember carefully combining that simple ingredients to observe small, controlled reactions—never anything dangerous, but just enough to fuel my interest. This hands-on experience deepened my love for chemistry and reinforced the idea that science isn’t just theoretical—it’s something you can see, hear, and feel.
I also recall one particular summer evening when I had the chance to safely assist in setting up a fireworks display under the supervision of professionals. It was an eye-opening experience—carefully handling the components, ensuring proper distances, and witnessing the precision required to create those brilliant bursts of color. It was then that I realized chemistry wasn’t just something in textbooks; it was alive, powerful, and breathtakingly beautiful. That experience played a big role in sparking my interest in chemistry and led me to explore the science behind these mesmerizing explosions.


Personal photos
The Science Behind Fireworks
Today, fireworks are made using rockets filled with gunpowder and metal compounds that create vibrant colors when ignited. These rockets must be tested in controlled environments to ensure safety, as fireworks can be highly dangerous if mishandled.
Each fireworks rocket contains several basic ingredients:
•Fuel: Gunpowder, a mixture of potassium nitrate (75%), sulfur (10%), and charcoal (15%).
•Oxidizing agents: These chemicals provide oxygen to sustain combustion. Common oxidizers include nitrates, chlorates, and perchlorates.
•Metal salts: These compounds give fireworks their distinct colors.
•Binder: Typically dextrin, a polysaccharide that slows and stabilizes the burn process.
Where Do Fireworks Get Their Colors?
The stunning colors in fireworks come from specific metal salts that burn at different wavelengths of light. Here are some of the most common:
•Red: Strontium salts (strontium nitrate, strontium carbonate, strontium sulfate, and strontium chloride).
•Blue: Copper salts, such as copper(I) chloride.

pictures: stock.adobe.com
•Orange: Calcium salts (calcium carbonate, calcium chloride, calcium sulfate).
•Yellow: Sodium salts (sodium bicarbonate, sodium nitrate, sodium chloride).

pictures: stock.adobe.com
•Green: Barium salts (barium nitrate, barium carbonate, barium chloride, barium chlorate).

pictures: stock.adobe.com
•Purple: A mix of copper and strontium salts.
•White/Silver: Produced by burning elemental metals like magnesium, aluminum, and titanium.

pictures: stock.adobe.com
The Beauty of Fireworks
Beyond the science, there is an undeniable artistry in fireworks. The combination of chemistry and physics creates a spectacular sensory experience. Whether they light up a festival, a New Year’s celebration, or a national holiday, fireworks have the power to bring people together, filling the sky with vibrant hues and awe-inspiring patterns.

Personal photo
Congratulations @dorianblack! You have completed the following achievement on the Hive blockchain And have been rewarded with New badge(s)
Your next target is to reach 50 comments.
You can view your badges on your board and compare yourself to others in the Ranking
If you no longer want to receive notifications, reply to this comment with the word
STOPCheck out our last posts: