We already know the problems derived from the use of fossil fuels, although the electrification of the transportation system is one of the best proposals to curb global warming, we are still far from having the infrastructure for the massive use of electric vehicles, however we can continue using the existing infrastructure using alternative liquid fuels, such as bioethanol, although this fuel is controversial because it competes with food production for the use of arable land. But a group of researchers have come up with an interesting proposal. Inspired by the power of photosynthesis, they have developed a method to convert CO2 and water, with the help of sunlight, into multi-carbon liquid fuels that can be used directly in a car engine.
Sunlight could help to produce liquid fuels. Source: pixnio, CC0.

And this type of fuels, although also based on carbon compounds, unlike fossil fuels, are completely renewable and have a zero net carbon emissions balance, since they are obtained from atmospheric carbon. And unlike bioethanol, which is obtained from cereals such as corn, or cellulose-rich plants such as sugar cane, it does not divert the use of any agricultural crop that could well be used in food production.
A research group from the Department of Chemistry at the University of Cambridge has sought to develop carbon-neutral fuels as they are considered to be cleaner and aim to play an important role in the transition to a more sustainable model. By carbon-neutral fuels we mean fuels that during combustion produce carbon dioxide emissions comparable to the carbon dioxide captured from the atmosphere for their production. And among them we can consider two types, those that have no carbon, such as hydrogen, and those that do, such as bioethanol.
factory and the car are public domain images.Carbon neutral fuels are considered cleaner. Source: @emiliomoron, the
In the research, the scientists assembled artificial leaf-like devices by integrating an oxide-derived Cu94Pd6 electrocatalyst with perovskite-BiVO4 light absorbers capable of inducing and combining CO2 reduction reactions with water oxidation. They designed a wireless device that produced ~1 µmol cm-2 of multicarbon alcohols (~1:1 ethanol and n-propanol) after 20 h operation under air irradiation.
This group of scientists has recreated the power of photosynthesis to harness CO2 and convert it into useful products, in this case, multicarbon fuels (ethanol and propanol). The advantage of this type of fuel is its high energy density, as well as the ease with which it can be transported and used directly in vehicle engines.
The most novel aspect of the research is that the researchers developed a copper and palladium catalyst, optimized so that the artificial leaves can produce complex chemical compounds, such as ethanol and n-propanol, in a single step.
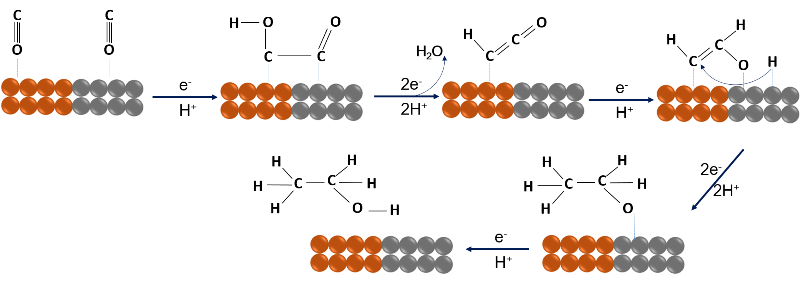
Schematic of ethanol formation over the copper-palladium catalyst. Source: image elaborated in powerpoint.
Although other researchers have succeeded in producing complex liquid substances similar to these with the use of electricity, this would be the first time that solar energy is used to produce them from CO2 and water, not to mention that in other works synthesis gas is obtained as an intermediate, while here they have been able to produce a usable liquid fuel in a single step.
Although at the moment the research is in its development phase, and only proofs of concept have been carried out with a very modest regiment, it certainly represents an important step in bringing us closer to the production of liquid fuels or value-added substances by using solar energy and CO2 captured directly from the air.
Hopefully, research will continue and in the future conditions can be optimized to obtain the volumes needed to scale up the technology and help limit dependence on fossil fuels.
Well friends, I hope you liked the information about this method to convert CO2 into liquid fuels. See you next time!
References
Rahaman, M., Andrei, V., Wright, D. et al. Solar-driven liquid multi-carbon fuel production using a standalone perovskite–BiVO4 artificial leaf. Natural Energy (2023).
Isn't it crazy to think that photosynthesis already accomplishes this? Nature is amazing.
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
You may also include @stemsocial as a beneficiary of the rewards of this post to get a stronger support.