Hello, dear readers. Today, I will be the discussing on the structure and bonding of the elements. I will first introduce the topic by explaining the discovery and structure of the fullerene and then go ahead to explain the structures of some other elements.
Fullerenes
The United States pavilion at the 1967 World Fair Expo in Montreal was a giant geodesic dome, a revolutionary structure designed by the pioneering American engineer and architect Robert Buckminster Fuller.
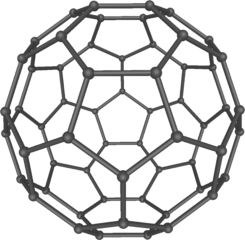
Model of the C60 fullerene (buckminsterfullerene). Mstroeck , CC BY-SA 3.0
Little did he know that 18 years later his design was to be repeated at atomic level in the Nobel Prize-winning discovery of forms of carbon that made up the class of structures named after him – the buckminsterfullerene These are net-like spheres (and tubes), each of many atoms, possibly opening up new areas of organic chemistry.
Until Harry Kroto and his team at Sussex University discovered the chemical class of fullerenes in 1985, chemists believed that carbon had only two crystalline forms: graphite and diamond. Now there were three. Scientists were astonished, because – so they thought – carbon had been exhaustively researched over many decades. Equally astonishing was the discovery that fullerenes are present in soot.
Like Buckminster Fuller’s dome, the design of which is held in place by natural forces, the structure of a crystalline form such as a fullerene is constrained by the forces that hold its component particles in place. It is this definite internal arrangement that determines the physical properties of all elements in the crystalline state.
STRUCTURE AND THE PERIODIC TABLE
From here you can read how elements in the same group of the Periodic Table have similar chemical properties because they have a similar arrangement of their outer electrons. For example, all the elements in Group 7 have an outer electron configuration that ends in p5. The periodicity of properties, such as ionization energy, is explained in terms of the regular recurring pattern of electron configurations from one period to another.
This article takes certain physical properties of elements and relates them to the structure and bonding of elements in the solid state. I will look particularly at the electrical conductivity, melting points and boiling points of the elements in the second and third periods of the Periodic Table.
KINETIC-MOLECULAR MODEL FOR SOLIDS
In any discussion of solid state structures we need to look at the kinetic molecular model of matter as it applies to solids. The figure below shows a typical arrangement of the particles in a solid. These are their common characteristics:
- The particles may be ions, atoms or molecules.
- They are closely packed in an ordered pattern, occupying relatively fixed positions.
- This ordered arrangement of particles is called a lattice.
- The particles in a solid are fixed in position, but are able to vibrate.
- The particles in a solid are attracted to one another.
I will look at the nature of some of the attractive forces later in the post.
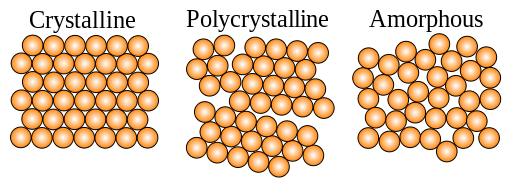
TYPES OF SOLID
When a liquid substance is cooled sufficiently, it freezes and forms a solid. Normally, the particles of the substance take up ordered positions and form a crystalline solid. Even elements that are gases at room temperature and pressure solidify when the temperature is low enough. For helium, the temperature has to be lowered to within 3 degrees of absolute zero before it becomes a solid.
Crystalline solids
In a crystalline solid, the particles are arranged in a form of definite repeating pattern throughout the solid. Such a solid is said to be homogeneous. This three-dimensional repeating pattern of particles in crystals is usually referred to as a crystal lattice.
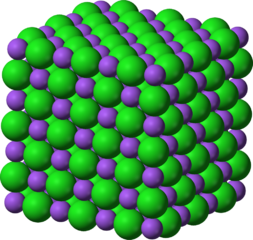
Many elements form crystalline solids and have a crystal lattice that can be described geometrically. An example is diamond, one of the allotropes of carbon. A crystalline solid has a definite melting point because all the attractive forces that hold the particles in the lattice have the same strength. So exactly equal amount of energy is required to overcome the forces between every pair of neighbouring particles.
Amorphous solids
Some elements do not always form crystalline structures. They form amorphous solids. Carbon black or soot is an example. In an amorphous solid, the particles do not take up a definite regular pattern during freezing. This is usually the case when the freezing process is so fast that the particles have no time to become arranged in an orderly way.
Metallic glasses are amorphous solids, formed when liquid metal cools at a rate of a million degrees per second. The particles occupy completely random positions within the amorphous solid and so do not form a crystal lattice. Nevertheless, the positions of the particles are relatively fixed.
Usually, amorphous solids don’t have a definite melting point, but soften gradually on heating. This is because the forces that hold their particles together have different strengths, and so different amounts of energy are needed to break them.
An element with an amorphous form always has at least one crystalline structure that is more stable than the amorphous form. Hence, an amorphous solid will slowly change into a crystalline solid. In this change of form, the particles are rearranged from a disordered state into an ordered pattern. However, if the forces between the particles in the amorphous form of a solid are strong, it could take a very long time to change from its amorphous form to its crystalline form.
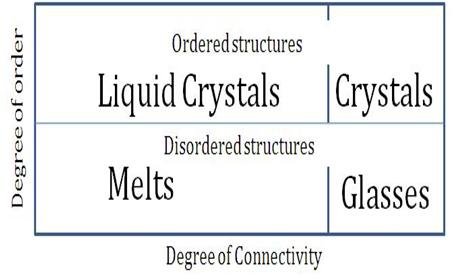
States of crystalline and amorphous materials as a function of connectivity. Ojovan, CC BY-SA 3.0
PATTERNS ACROSS THE PERIODIC TABLE
I have already described the periodicity in properties, such as first ionization and atomic radii, that depend on the electron configuration of the elements. The periodicity shown by the physical properties of elements, such as boiling point or electrical conductivity, depends on the structure and bonding rather than the electronic configuration.
MELTING AND BOILING POINTS
The melting points and boiling points of elements vary with atomic number (proton number). Although there is a pattern – the melting point tends to rise to a maximum around Group 4 and then falls to the noble gases – it cannot really be described as a periodic function. In fact, if the melting point graph extended to elements with higher atomic numbers, we would see even less of a pattern.
Put simply, it is the strength of the attraction between the particles in an element’s crystal lattice that determines the value of the melting point, rather than the electron configuration of the element. The greater the force between the particles, the higher the melting point. Much the same can be said of the boiling point, but in this case it is the magnitude of the attraction between the particles in the liquid phase.
In terms of the melting and boiling points, there are broadly three types of element:
- Metals that have reasonably high melting points:
- Non-metals that have very high melting points (such as carbon and silicon):
- Non-metals that have very low melting points.
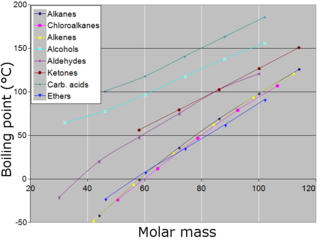
The difference in the melting points of elements depends on their crystal structure and the strength of the bonding between particles (atoms, molecules or ions) of the elements.
ELECTRICAL CONDUCTIVITY
Metals are good electrical conductors and non-metals are poor electrical conductors. For electrical conduction to occur, there must be charged particles that are free to move. In the case of an element, the charged particles are its electrons. Provided electrons in an element are free to move when subjected to a potential difference (i.e. when a voltage is applied), the element is a good electrical conductor. There is a complication: the electrical conductivity of elements can change under varying conditions of temperature and pressure.
METALLIC AND NON-METALLIC ELEMENTS
We can understand the differences in the melting points, boiling points and electrical conductivities of the elements by looking at the types of particle which make up the crystal lattices.
A metal crystallises into a giant structure of closely packed metal ions. No molecules are present. By contrast, a typical non-metal is very different: its structure normally contains covalent bonds, so it consists of individual molecules. Hence, the arrangement of particles in a non-metal is described as a molecular lattice.
The molecules may be giant, and we can consider the whole crystal as being one molecule, as in diamond and graphite. Alternatively, the lattice may be made up of repeating units of simple molecules, as in the case of solid iodine, which has a lattice that contains a repeating pattern of iodine molecules, I2. The noble gases are monatomic, so the crystal lattice of a solid noble gas is based on a repeating pattern of atoms.
Across the whole range of crystalline elements, there is a wide variation in the strengths of the attractive forces between the particles that form their different crystal structures. Evidence for this is the wide variation in the melting and boiling points of these elements shown in the figure above. Solids with small molecules, such as hydrogen, nitrogen and oxygen, have low melting points because the forces between them in the crystalline state are very weak. Graphite and diamond have very strong forces of attraction – covalent bonds – between their atoms, which gives them extremely high melting points.
METALLIC BONDING
Look at a lump of any pure metallic element. It is not obvious that it is crystalline: it does not have the regular faces that a crystal of diamond has. Nevertheless, a powerful microscope reveals its crystalline structure.
We can see crystals forming in the displacement reaction between zinc and aqueous silver nitrate or lead(II) nitrate:
Zn(s) + 2Ag(NO3)2(aq) → Zn(NO3)2(aq) + 2Ag(s)Zn(s) + Pb(NO3)2(aq) → Zn(NO3)2(aq) + Pb(s)
CLOSE PACKING
The existence of crystals in a piece of metal means that the particles in the metal must have a regular arrangement. These particles are, in fact, positive ions and not metal atoms.
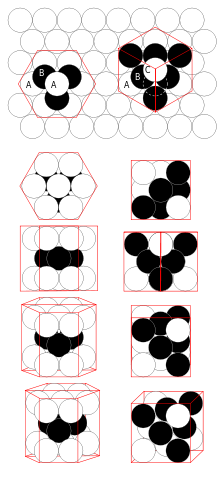
All of these ions are the same size, so they can close pack in a manner similar to the most space-saving way of packing spheres in a layer: each sphere is surrounded by six others.
In some metals, close packing is achieved through a repeating pattern in which each positive ion is in contact with six others in the same layer. Such a crystal will have not just one but many layers of positive ions. Each additional layer is arranged to maximise the number of ions that can be packed.
However, there is more than one way of close-packing positive ions in a three-dimensional metallic crystal lattice. The coordination number is the number of nearest neighbours that a particle has when it is in a crystal lattice.
A unit cell is the simplest pattern of particles for which repetition in three dimensions produces a crystal lattice. Since metal ions are closely packed, it is not surprising that most metals have high densities.
POSITIVE IONS IN A SEA OF DELOCALISED ELECTRONS
Since they all have the same charge, why don’t the metal ions repel one another? Clearly, they don’t, otherwise metals would not be strong and mostly hard. The answer lies in the electrons that have been removed to form the positive ions. These electrons are free to move throughout the metal crystal. The electrons are said to be delocalised. The delocalised electrons are often referred to as a ‘sea of electrons’, because they occupy the whole space between the closely packed metal ions. The positive metal ions are not repelled from one another because each is electrostatically attracted towards the sea of delocalised electrons.
REFERENCES
https://www.sciencemag.org/news/1996/10/fullerene-discoverers-win-chemistry-nobel
https://www.britannica.com/science/fullerene
https://en.wikipedia.org/wiki/Fullerene
https://www.mikeblaber.org/oldwine/chm1045/notes/Forces/Kinetic/Forces01.htm
https://study.com/academy/lesson/the-kinetic-molecular-theory-properties-of-solids-and-liquids.html
https://courses.lumenlearning.com/boundless-chemistry/chapter/kinetic-molecular-theory-of-matter/
https://www.imedpub.com/scholarly/crystalline-solids-journals-articles-ppts-list.php
https://en.wikipedia.org/wiki/Crystal
https://www.toppr.com/content/concept/amorphous-and-crystalline-solids-203675/
https://en.wikipedia.org/wiki/Amorphous_solid
https://www.britannica.com/science/amorphous-solid
https://en.wikipedia.org/wiki/Periodic_table
https://www.bbc.co.uk/bitesize/guides/zxc99j6/revision/6
https://en.wikipedia.org/wiki/Periodic_trends
https://www.thoughtco.com/metals-versus-nonmetals-608809
https://en.wikipedia.org/wiki/Nonmetal
https://courses.lumenlearning.com/cheminter/chapter/metallic-and-nonmetallic-character/
https://www.siyavula.com/read/science/grade-10/chemical-bonding/06-chemical-bonding-05
https://www.bbc.co.uk/bitesize/guides/zqmrsrd/revision/6
https://courses.lumenlearning.com/introchem/chapter/bonding-in-metals-the-electron-sea-model/
Thanks empressteemah for this educative piece. A little summary at the end will also be fine for people that are not chemistry oriented. Keep up with the good work.
Alright, sir. Thanks for coming by.
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider supporting our funding proposal, approving our witness (@stem.witness) or delegating to the @steemstem account (for some ROI).
Thanks for using the STEMsocial app
, which gives you stronger support. Including @steemstem as a beneficiary could yield even more support next time.