Hello, great readers. Here is a continuation from where I stopped in my last post on Esters. Have a quick read of the two posts here and here. But, today, I will start by explaining the Acyl chlorides and their reactions, and then go ahead to explaining more about acid anhydride, amides and nitriles.
ACYL CHLORIDES
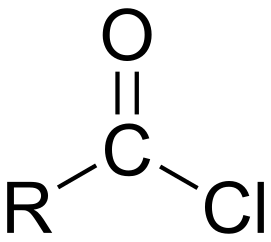
An acyl chloride is a carboxylic acid in which the hydroxyl group has been replaced with a chlorine atom. Acyl chlorides are highly reactive compounds that make very useful synthetic intermediates. Acyl chlorides can be prepared by reacting sulfuryl (IV) chloride, SOCI2, or phosphorus(V) chloride, PCl5, with carboxylic acid. Acyl chlorides are named after the corresponding carboxylic acid, using suffix -oyl followed by chloride. For example, CH3COCI is ethanoyl chloride and C6H5COCI is benzoyl chloride.
ADDITION-ELIMINATION REACTIONS OF ACYL CHLORIDES
An acyl chloride has an extremely electron-deficient carbonyl carbon because of the electron-withdrawing effect of the highly electronegative chlorine atom. This enhances the reaction of a nucleophile with the carbonyl carbon. Nucleophilic addition occurs and the carbonyl double bond is broken. The addition product then eliminates the chloride as an ion to regenerate the carbonyl double bond. This mechanism is shown below. The curly arrows show the movement of an electron pair.
The addition-elimination reaction is an example of a condensation reaction, in which two molecules react together with the elimination of a simple molecule, such as water, hydrogen chloride or an alcohol. With an acyl chloride, hydrogen chloride is eliminated.
HYDROLYSIS OF ACYL CHLORIDES
An acyl chloride reacts readily with water to form the corresponding carboxylic acid. Most acyl chlorides fume in air because of this reaction with water vapour: the hydrogen chloride gas formed dissolves in atmospheric moisture to form tiny droplets of hydrochloric acid. For instance, ethanoyl chloride fumes in moist air to form ethanoic acid and hydrogen chloride. In water, hydrochloric acid would be formed instead. This reaction of the carbon-chlorine bond is much more rapid than reactions involving other carbon-chlorine bonds. The highly electron-dence carbonyl carbon encourages nucleophilic addition, to be followed later by elimination. When an acyl chloride is hydrolysed in alkaline solution, the carboxylate ion is formed instead of the acid.
Preparation of esters from acyl chlorides
The reaction of a carboxylic acid with an alcohol is an equilibrium process, which makes it difficult to obtain good yields of esters. An alternative way to make an ester from a carboxylic acid involves the formation of an acyl chloride and the subsequent reaction of the acyl chloride with an alcohol. Acyl chlorides react completely with alcohols and do not form an equilibrium mixture. This provides, in the laboratory, a powerful synthetic route to make esters. Both steps of the process can be achieved with yields over 90 per cent.
The addition-elimination reaction between acyl chlorides and alcohols
Chemists use models to explain chemical reactions. The ‘curly arrow’ model, the movement of electron pairs, is a powerful way of explaining reaction mechanisms. Any chlorides react with alcohols and phenols to form esters. These reactions have a mechanism known as nucleophilic addition-elimination.
In the reaction between an acyl chloride and an alcohol the first stage is nucleophilic addition of the alcohol. An alcohol molecule can act as a nucleophile by donating a one pair of electrons from the oxygen atom to the electron-deficient carbonyl carbon atom, thereby forming a covalent bond.
The mechanism’s next stage involves the transfer of a proton from one highly electronegative atom to another highly electronegative atom. Following proton transfer, hydrogen chloride is eliminated. When the reaction is carried out in the presence of an alkali, the hydrogen chloride reacts to form a chloride ion and it becomes impossible for the reaction to be reversed.
Phenol reacts with acyl chlorides in the same way, and the presence of an alkali can assist the reaction in a second way. Phenol is sufficiently acidic for a reaction with an alkali, such as aqueous sodium hydroxide, to form the phenoxide ion, C6H5O-. This ion is a much better nucleophile than the neutral phenol molecule, and so the first stage of the reaction, nucleophilic addition, occurs easily.
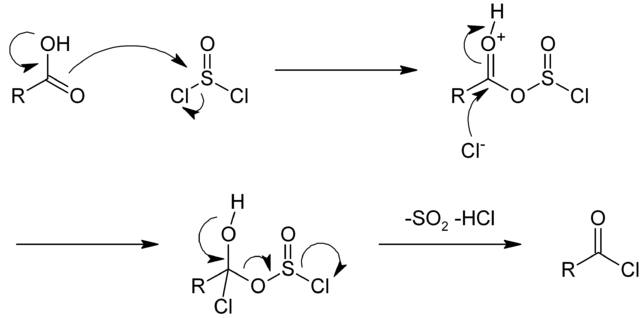
Action of en:thionyl chloride on en:carboxylic acids. Rifleman 82, Public Domain
Reactions of acyl chlorides with ammonia and amines
Ammonia and other amines are nucleophiles because they have a lone pair of electrons on the nitrogen atom. They can react with acyl chlorides in the same way as alcohols. This time, the products formed are amides. Amides are difficult to make from carboxylic acids, since carboxylic acids react with ammonia in an acid-base reaction to form an ammonium salt instead of an amide. So, the use of an acyl chloride as a synthetic intermediate solves this problem. And it is easy to produce amides by the reaction of an amine or ammonia with an acyl chloride. It is normal to use an excess of the amine or ammonia, so that the excess is available to remove the hydrogen chloride eliminated at the end in an acid-base reaction.
Ethanoyl chloride reacts with excess ammonia to make ethanamide. Notice from this equation that the excess of ammonia leads to the formation of ammonium chloride. Ethanoyl chloride also reacts with methylamine to make N-methylethanamide.
ACID ANHYDRIDES
Acid anhydrides are very similar to acyl chlorides and are also very useful synthetic intermediates. Acid anhydrides can be prepared by distilling a sodium carboxylate with an acid chloride. So sodium ethanoate and ethanoyl chloride will form ethanoic anhydride and leave behind sodium chloride as an involatile salt.
Acid anhydrides are normally named after the corresponding acid, so that (CH3CO)2CO is ethanoic anhydride.
REACTIONS OF ACID ANHYDRIDES
Nucleophiles such as water, hydroxide ions, ammonia, alcohols and amines react with acid anhydrides by an addition-elimination mechanism with the elimination of a carboxylic acid. A typical reaction is that of ethanoic anhydride with phenol to make phenyl ethanoate.
Manufacture of aspirin
Aspirin is a well-known painkiller. It is made from phenol in a two-step process. The formation of the ester linkage in stage two of the synthesis uses ethanoic anhydride. 2-hydroxybenzoic acid and ethanoic anhydride are refluxed together and the aspirin is precipitated by placing the reaction mixture in water. The aspirin can then be filtered and purified before it is ready to be sold commercially. Ethanoic anhydride is used to synthesise the ester rather than ethanoyl chloride, since ethanoic acid (the co-product formed) is less volatile than hydrogen chloride (the co-product with ethanoyl chloride). Another advantage with ethanoic anhydride is that it is much cheaper to manufacture than ethanoyl chloride.
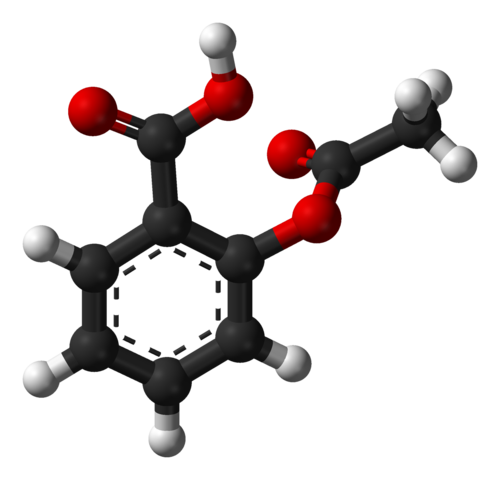
Ball-and-stick model of the aspirin molecule, as found in the solid state. Ben Mills, Public Domain
AMIDES
Amides have an amino group directly attached to the carbonyl carbon. Proteins, polypeptides and synthetic polymers such as nylon contain the amide linkage.
NAMING OF AMIDES
Amides are named by adding the suffix amide to the name of the carbon skeleton. Take, for example, ethanamide, pentanamide and benzamide. Ethanamide is a two-carbon chain in which the number 1 carbon is part of the amide functional group. Its formula can be written as CH3CONH2. Pentanamide is a five-carbon chain in which the number 1 carbon is part of the amide group. Benzamide consists of a benzene ring attached to an amide functional group. It has the formula C6H5CONH2.
These amides are known as primary amides, because they have only one carbon atom attached to the nitrogen atom of the amide. Secondary amides have two carbon atoms attached, and tertiary amides have three carbon atoms attached.
Note that the extra carbon atoms are not part of the carbon chain that contains the carbonyl group, so they are considered as substituents. Their position is indicated by N-, which signifies they are bonded to the nitrogen atom. For example, N-methyl ethanamide has the formula CH3CONHCH3. It has a two-carbon chain, which includes the carbonyl carbon, and a methyl group, which is attached to the nitrogen atom.
REACTIONS OF AMIDES
Amides show the same types of reaction as the other derivatives of carboxylic acids, but they are rather less reactive.
Hydrolysis of amides
The reaction can be catalysed by an acid or a base. For example, just as with esters, the reaction of an amide with water is extremely slow, but on hydrolysed using boiling hydrochloric acid, ethanamide forms ethanoic acid and ammonium chloride. When refluxed with aqueous sodium hydroxide, it forms sodium ethanoate and ammonia.
NITRILES
Nitriles are not acid derivatives, but they do behave similarly to acid derivatives because of the presence of a polar carbon-nitrogen triple bond. They are important synthetic intermediates and are often involved in synthetic routes that need to extend the carbon skeleton.
NAMING OF NITRILES
Nitriles are named by adding the suffix ‘nitrile’ to the parent alkane. So CH3CN would be called ethanenitrile and CH3CH2CN would be propanenitrile.
PREPARATION OF NITRILES
Nitriles are normally prepared using hydrogen cyanide, HCN, or the cyanide ion, CN-. Either nucleophilic substitution of a halogenoalkane using CN-, or nucleophilic addition of HCN to an aldehyde or ketone will make a nitrile.

The structure of a nitrile: the functional group is highlighted blue. Jü, Public Domain
Nucleophilic substitution of halogenoalkanes
I’ve described in a recent post of mine on how halogenoalkanes can react by nucleophilic substitution with potassium cyanide using ethanol as a solvent – so propanenitrile can be prepared by reacting bromoethane with ethanolic potassium cyanide. Notice that in this reaction the carbon skeleton has becn extended by one carbon.
Nucleophilic addition of aldehydes and ketones
In the reaction of HCN, in the presence of CN-, with aldehydes and ketones. This reaction made a cyanohydrin, which is a hydroxynitrile; so propanal will react with HCN to make 2-hydroxybutanenitrile. Notice that in this reaction the carbon skeleton has again been extended by one carbon.
REACTIONS OF NITRILES
Nitriles have similar properties to carbonyl compounds, because both have polar multiple bonds: the nitriles have a triple bond and carbonyl compounds a double bond. The carbon atom of the CN group is susceptible to attack by nucleophiles.
Hydrolysis of nitriles
Nitriles are hydrolysed extremely slowly by water, but the hydrolysis occurs much faster in either acidic or basic conditions. With acidic hydrolysis a carboxylic acid and an ammonium salt are formed. With basic hydrolysis a carboxylate and ammonia are formed.
Reduction of nitriles
Nitriles can be reduced by reaction with the powerful reducing agent LiAIH4 to form a primary amine. In the equation the reducing agent is represented by [H] to show that it provides hydrogen atoms for the reduction. Alternatively a nitrile can be hydrogenated by using hydrogen under pressure in the presence of a nickel catalyst.
SYNTHESIS INVOLVING NITRILES
Nitriles are often used as synthetic intermediates. Ethanal can be converted into 2-hydroxypropanoic acid, which most people would more easily remember by its trivial name of lactic acid. The synthetic intermediate is 2-hydroxypropanenitrile.
SUMMARY
After going through this post and the previous ones I've written on this topic (which are this and this), you should know that:
- Esters, acyl chlorides, acid anhydrides and amides are all derivatives of carboxylic acids. Esters can be prepared by refluxing a carboxylic acid and an alcohol in the presence of a small amount of concentrated sulfuric acid as a catalyst. Esterification is a reversible reaction that reaches a dynamic equilibrium. At equilibrium, the concentrations of all the substances involved remain constant, and the rate of the forward reaction is equal to the rate of the backward reaction.
- Le Chatelier’s principle states that the position of equilibrium changes to minimise any change imposed on the conditions at equilibrium. The equilibrium constant for a reversible reaction is the product of the molar concentrations of the products, each raised to the power of its coefficient in the stoichiometric equation, divided by the product of the molar concentrations of the reactants, each raised to the power of its coefficient in the stoichiometric equation. The numerical value of the equilibrium constant only changes if the temperature of the equilibrium process changes.
- Acyl chlorides and acid anhydrides react with alcohols to form esters in reactions that go to completion. All derivatives of carboxylic acids react by nucleophilic addition-elimination (i.e. by condensation reactions). Acyl chlorides and acid anhydrides can be hydrolysed by water to give the corresponding carboxylic acid; whereas amides and esters need to be hydrolysed using an acid or a base catalyst.
- Amides can be made by the reaction between acid anhydrides or acyl chlorides and ammonia or amines. Nitriles are good synthetic intermediates because their formation involves the extension of the carbon skeleton. Nitriles can be hydrolysed to make carboxylic acids and reduced to make primary amines.
Thanks for reading.
REFERENCES
- https://study.com/academy/lesson/acyl-chloride-reactions-synthesis.html
- https://www.chemguide.co.uk/organicprops/acylchlorides/background.html
- https://www.chemguide.co.uk/organicprops/acylclmenu.html
- https://en.wikipedia.org/wiki/Acyl_chloride
- https://en.wikipedia.org/wiki/Acid_anhydride
- https://www.khanacademy.org/test-prep/mcat/chemical-processes/carboxylic-acids/a/carboxylic-acid-reactions-overview
- https://www.chemguide.co.uk/organicprops/anhydrides/oxygen.html
- https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Map%3A_Organic_Chemistry_(Smith)/Chapter_22%3A_Carboxylic_Acids_and_Their_Derivatives%E2%80%94_Nucleophilic_Acyl_Substitution/22.9%3A_Reactions_of_Anhydrides
- http://www.madehow.com/Volume-1/Aspirin.html
- https://www.sciencedirect.com/topics/chemistry/acetylsalicylic-acid
- https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Map%3A_Organic_Chemistry_(McMurry)/21%3A_Carboxylic_Acid_Derivatives-_Nucleophilic_Acyl_Substitution_Reactions/21.08%3A_Chemistry_of_Amides
- https://www.chemguide.co.uk/organicprops/amides/other.html
- https://study.com/academy/lesson/amides-reactions.html
- https://en.wikipedia.org/wiki/Amide
- https://study.com/academy/lesson/nucleophilic-substitution-in-haloalkanes.html
- https://www.cliffsnotes.com/study-guides/chemistry/organic-chemistry-ii/aldehydes-and-ketones/reactions-of-aldehydes-and-ketones
- https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Map%3A_Organic_Chemistry_(McMurry)/19%3A_Aldehydes_and_Ketones-_Nucleophilic_Addition_Reactions/19.04%3A_Nucleophilic_Addition_Reactions_of_Aldehydes_and_Ketones
- https://study.com/academy/lesson/nucleophilic-addition-reactions-of-aldehydes-ketones.html
- https://pubs.acs.org/doi/10.1021/acs.joc.8b02190
- https://www.organic-chemistry.org/synthesis/C3N/nitriles.shtm
- https://en.wikipedia.org/wiki/Nitrile
- https://en.wikipedia.org/wiki/Letts_nitrile_synthesis
Not really a fan of organic chemistry but I'm sure those that fancy it will find this interesting.
You have been contributing well to the community over the years and I will really love to see your participation within the community transcends just writing posts. Feel free to read other people's posts and engage them in the comment section.
Thanks for being a consistent author in the community.
Thanks for this prompter. You are actually right and I promise to surely adjust.
Congratulations @empressteemah! You have completed the following achievement on the Hive blockchain and have been rewarded with new badge(s) :
You can view your badges on your board and compare to others on the Ranking
If you no longer want to receive notifications, reply to this comment with the word
STOPDo not miss the last post from @hivebuzz:
Vote for us as a witness to get one more badge and upvotes from us with more power!
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider supporting our funding proposal, approving our witness (@stem.witness) or delegating to the @steemstem account (for some ROI).
Thanks for using the STEMsocial app
, which gives you stronger support. Including @steemstem as a beneficiary could yield even more support next time.