Microbial co-habitation and collaboration underpin a complex flavour profile

Photo by Thomas Thompson on Unsplash
If humanity can claim any progress in any realm of its existence, it is in the sciences. There is little question that science and technology have revolutionized our understanding of the world, let alone our beers.
In contrast to most beer styles, we know little about the processes that give Lambic beers their distinct character. While we know the general production process used in Lambic brewing, the biochemistry of fermentation and ageing is a bit of a black box. The critical question is not, how “do they make them?”. The more important question here is; what exactly happens during the fermentation and ageing process?
We already know that Lambic beer production involves the natural seeding of sterile wort. Wort exposure leads to invasion and colonization by a diverse microbial community present in the environment. Subsequent growth, competition and successive fermentation and conversion steps lead to the production of alcohol, acids and a wide range of metabolites that impart the distinct flavour and aroma profiles found in this beer. Given the complexity of the beer and process, it may seem impossible to disentangle processes.
The emergence of cheap genome sequencing technologies, along with advanced computer algorithms that can make sense of sequence data sets, have revolutionized experimental biology. Now, it is not only possible to sequence a genome in a matter of days and analyze it in a couple of weeks, but we also can sequence all the organisms in a sample and generate a “meta-genome”, the combined genome of a diverse population. Once you have a meta-genome, you can identify the genes present in the population. In doing so, you can generate a picture of population-level functionality. What does the microbial genome look like and what can it do (biochemically)?
By now, you can probably already see how we can apply such technology to the “Lambic Beer Problem”. Let’s sequence whatever microbe is in the beer and sort this meta-metabolic network stuff.
The paper that I discuss below has done that and much more.
While sequencing the microbiome of a finished Lambic beer has its attractions, the insights gained from such an approach would be very limited. If your hypothesis is that microbial composition is static (does not change) throughout the production process, it would be ok. But with Lambic beers, where fermentation is complex and involves dynamic changes to the substrate and microbial community, we require a more sophisticated approach.
With the temporal aspects of Lambic fermentation in mind, Jonas De Roos, Marko Verce, Stefan Weckx and Prof. Luc De Vuyst designed a time-course experiment in which they sampled Lambic wort and beer in various stages of fermentation and ageing, respectively. Using the four broadly defined stages of fermentation and maturation (the enterobacterial phase, the alcoholic fermentation phase, the acidification phase, and the maturation phase), they isolated microbes three days, three weeks, three months, nine months, 13 months and 24 months after the transfer of cooled wort into wooden barrels.
Critically, DNA used for sequencing was prepared directly from the isolated microbes, preventing any intermediate culturing steps. They then sequenced the resulting samples using a mass parallel sequencing approach on an Ion-Torrent system. Using this approach, the researchers generated nearly 3.7 billion base pairs (bp) across the six samples. Critically, individual reads (sequences) are up to 400 bp long, requiring significant bioinformatic effort to put together into larger fragments (contigs). When you do, however, not only can you use such sequences to trace back its origin (which microbe did it come from), but also, what genes are on those large contigs and what could those functions be? By compiling such information, the authors could catalogue both the microbial composition and gene content in each of the fermentation stages.
What did they find?
As you may expect, there were a wide range of organisms present at varying relative levels. They found representatives for the following genera in (from high to low relative levels):
Yeasts (from high to low relative abundance):
- Saccharomyces (60-90% of reads in the first couple of weeks)
- Brettanomyces (Dominant (>90%) at the last stages)
- Pichia (Late-stage of fermentation/maturation)
- Hanseniaspora (only in day-3 and week-3 samples with low abundance)
- Ogataea (Late-stage of fermentation/maturation)_
- Candida (Late-stage of fermentation/maturation)
- Kuraishia (Late-stage of fermentation/maturation)
- Malassezia (Late-stage of fermentation/maturation)
- Wickerhamomyces (Late-stage of fermentation/maturation).
Bacteria (from high to low relative abundance):
- Acetobacter (Most prevalent in three-month-old samples)
- Pediococcus (increasing in the maturation phase)
- Komagataeibacter
- Lactobacillus (increasing in the maturation phase)
- Klebsiella (most prevalent at the start of fermentation)
What does this all mean?
On a very basic level, the composition of microbial communities dynamically changes. The early fermentation stage is dominated by Saccharomyces species and acetobacter representatives, whereas in the late stages, yeasts such as Brettanomyces and Pichia take over. This, of course, raises the question of what their potential roles in the fermentation process could be?
One way to address this is by assessing gene content. Bioinformatic analyses of sequences generated in this study could identify all the components required for the conversion of carbohydrates to alcohol, acetic acid and the breakdown of complex malto-sugars. Some enzymes required, for the conversion steps, could be identified across all species, whereas enzymatic steps or enzymes appeared to be species-specific. This is significant, as it shows that some species abundant in a particular stage may play specific ecological roles that benefit the overall community or process.
If certain microbes perform specific tasks to the benefit of the beer-ecosystem, one should be able to find associations between the appearance of a species and the (dis) appearance of (a) metabolite (s). One way to test this hypothesis is by looking for metabolic signatures that correlate with the presence of a microbe. They found negative correlations between Saccharomyces abundance and ethanol levels and the emergence of species, potentially responsible for the production of key phenolic compounds. In addition, meta genome analyses suggested that an as yet unidentified Acetobacter species contributes to the acidification of Lambic beers.
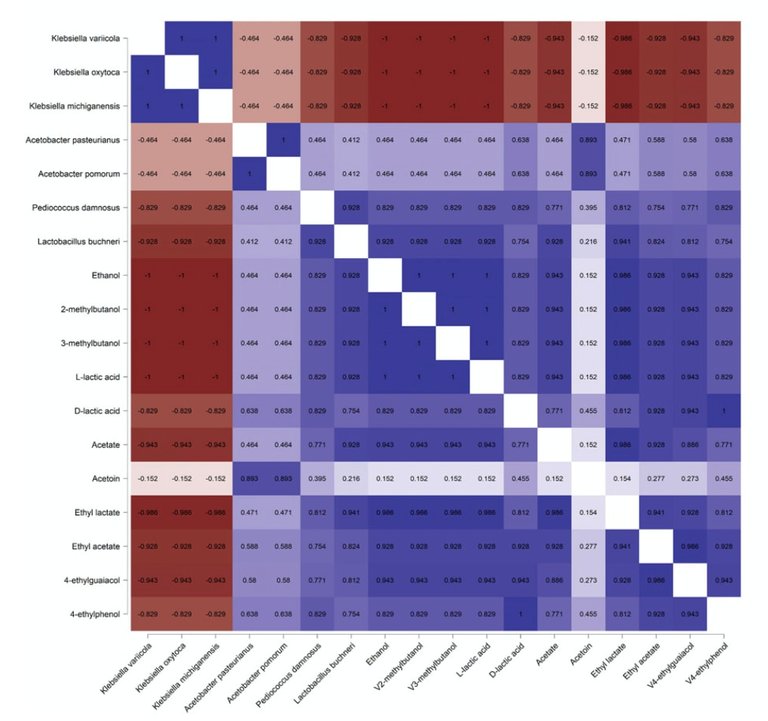
Figure 8B from De Roos et al., (2020). Correlation between microbes and compounds found during the fermentation and maturation process in Lambic beer. Red represents a negative correlation, whereas blue is positive. This data shows that the rise and disappearance of metabolic compounds are correlated with microbe abundance. In addition, correlations also help to establish relationships between different microbial genera.
What are the main takeaways from this work?
- As established previously, the production of Lambic beers involves a succession of microbial colonization or proliferation events, each associated with the known stages of fermentation/maturation. Interestingly, microbial diversity was relatively low, suggesting that wort (and beer in the later stages) offers a harsh environment in which only adapted microbes can survive and thrive.
- We can associate the emergence and disappearance of metabolic compounds to particular species, reinforcing the need for microbial succession in the production of Lambic beers.
- Not all metabolic steps and conversions were traced back to a particular organism or enzymes. These results suggest that sampling needs to be improved (more samples, more reps) or that some of these organisms carry genes that encode as yet unidentified enzymes and activities.
I hope you will now appreciate the complexity and beauty that underpins the Lambic beer (though I suspect that many of you already did!).
Please share or forward this article to anyone who may enjoy reading about this topic.
Best wishes,
Edgar, The Beerologist.
P.S. You can find the full paper here. Get in touch with me if you have any further questions.
***
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider supporting our funding proposal, approving our witness (@stem.witness) or delegating to the @stemsocial account (for some ROI).
Please consider using the STEMsocial app app and including @stemsocial as a beneficiary to get a stronger support.