Andreas Glöckner Authored by @madridbg, via Power Point 2010, using public domain images.
Greetings and welcome dear readers who accompany me daily in the exciting world of science and its implications in our daily lives. In this sense and following the structure of the contents socialized in this space, through this delivery we will be addressing what concerns the atmospheric pressure and how it influences in daily activities, taking as a reference the height between cities in relation to sea level.
Consequently, and as has been our daily practice, we will share this type of material through the communities of greatest scientific reference on the platform, mentioning @Stemsocial.
CONCEPTUAL APPROACH

Understanding the behavior of matter has been one of the activities in which man has emphasized during his historical development and although we live in an atmosphere based on a mixture of gases, it is easier to understand the functioning and dynamics of liquids and solids, since they are tangible and we are in daily contact with these substances.
Thus, we daily use water as a liquid substance to bathe, hydrate, wash or cook, in the same way, we usually manipulate solid materials that we use to sit, dress, among others, and in the case of gases we can only mention some tangible uses such as the gas used for cooking and the oxygen we breathe.
Now, if we compare the movement of the particles that make up these materials, we realize that in solids and liquids, the molecules are more rigid than in gases, due to a chemical basis called molecular packing, which explains the properties and behavior of the states of matter.
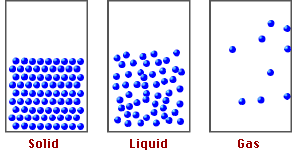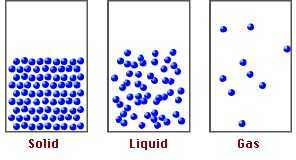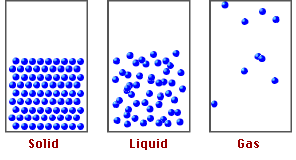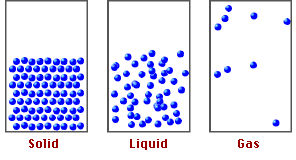
Fig. 2. Representation of the states of matter. Author: tecnicosistema17
Based on the above, it is the intermolecular forces that keep the atoms of the molecules in the compounds together and it is these forces that will allow us to understand how pressure at high altitudes can affect the functioning of matter.
APPROACH TO PHASE CHANGES

Chemically a phase change is assumed to be a transformation that occurs from one phase to another and usually occurs when energy is added or removed from the system in the form of heat, these changes are physical and can be distinguished due to the changing molecular order that is generated, knowing that during the solid phases the molecules have reached the maximum order and during the gaseous state the entropic levels or disorder increases in the molecules. In such a way that the changes generated between energy and molecular disorder caused by the phase change will allow us to understand the nature of these physical changes.
Among the different changes in relation to energy and molecular order, the following stand out:
1. Liquid-steam relationship: in simple words is the transformation of liquid substances into gaseous substances, this is achieved through a considerable energy change that allows a greater number of collisions between particles, which causes a greater kinetic energy in the system, which results in greater entropy or disorder, this state of excitation is what allows the liquid particles to escape from the container as steam. This process is called evaporation or vaporization.
In this sense, it is necessary to emphasize that this process is reversible and it is only necessary to extract energy from the system to recover the liquid phase originally present in the system, a process called condensation.
2. Liquid-solid relationship: this relationship is known by the terms freezing or melting, the first refers to the passage from a liquid to a solid state and the second is the opposite step, i.e., from solid to liquid. In this sense, for melting or freezing to occur, a dynamic equilibrium between the two phases must be formed within the system.
An example of the above is represented by the equilibrium formed between water and ice at 0°C and 1 atmosphere of pressure because as the ice cube melts some water molecules freeze, however, although the systems were at 0°C the water will not remain in time at that value, hence it is assumed as an equilibrium formed.
3. Solid-steam relationship: at the level of the chemical behavior of substances, solids also have the property of undergoing the evaporation process and consequently generate a vapor pressure when the dynamic equilibrium is formed between the solid and the vapor generated.
In this sense, the process in which molecules pass from the solid state to the gaseous state without passing through the liquid is known as sublimation, the opposite case being regressive sublimation. An example of this is dry ice or, alternatively, naphthalene.
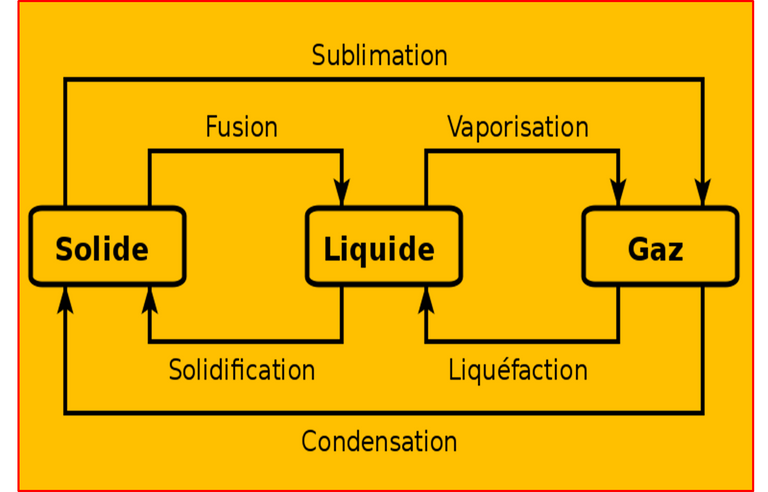
Fig. 3. Phase changes of matter. Author: AnyFile
Taking into account the behavior of the material as a function of the change of states, let us evaluate some everyday aspects where the pressure exerts direct influence on the behavior of materials, aspects that we will study next.
EVERYDAY ASPECTS RELATED TO PHASE CHANGES

In this section of the subject we will deal with some commonly used scenarios where we can observe phase changes and their relationship with matter, among which the following stand out:
1. About the process of cooking eggs on top of a mountain: as readers let's assume that we reach the top of a mountain above 4 meters above sea level and as a measure of rest we decide to camp and prepare our meal, which would be boiled egg, to surprise the water boils much faster but the egg takes longer in the cooking process, I dare to ask you why is this?

Fig. 4. Representation of boiling water. Author: Markus Schweiss
To answer this question it is necessary to take into account the phase equilibrium processes studied previously, where the atmospheric pressure at that altitude will be approximately 0.6 atmospheres, which is assumed to be a pressure lower than the normal 1 Atm, this relationship causes the boiling point of water to drop from 100°C to 86°C.
However, in the cooking process of any food, it is not the boiling point that is important, but the heat supply that is provided to it, at this point the egg will take approximately 30 minutes to be well cooked, a much longer time if compared at altitudes above sea level.
2. About ice skating: If we analyze the sports scenario of ice skating, at first glance we can determine that during the process there is an ice-water equilibrium. At this point, thanks to the sharp blades of the skates, a person of 80 kg exerts an atmospheric pressure equivalent to 500 atm on the surface of the ice, consequently at temperatures below 0 °C the ice under the blades melts and becomes liquid, creating a film of water that allows skating on the ice.

Author: Manfred Richter, Pixabay.
FINAL CONSIDERATIONS

As we have analyzed along these lines of writing, understanding the basics of chemistry allows us to have a broader view of the phenomena that occur in our daily lives, so that this type of content generates some scientific literacy in aspects associated with chemistry from a flexible perspective, so that users can stop seeing chemistry as an abstract discipline and consider it as the discipline that allows us to explain the why of things.
BIBLIOGRAPHY CONSULTED

[1] McMURRY E., John and Fay C., Robert (2008). General chemistry. Fifth edition PEARSON EDUCATION, Mexico, 2009 ISBN: 978-970-26 1286-5.
[2] Ralph, H. Petrucci, William S. Harwood, E. Geoffrey Herring (2003). GENERAL CHEMISTRY. Eighth edition. PEARSON EDUCACIÓN. S.A., Madrid.
OF INTEREST

1. The cover image was made by @madridbg, used public domain image.
2. For more information related to the areas of science, technology, engineering and mathematics, feel free to visit #stemsocial and #stem-espanol, communities that promote scientific advances in these areas.

The rewards earned on this comment will go directly to the people( @madridbg ) sharing the post on Twitter as long as they are registered with @poshtoken. Sign up at https://hiveposh.com.
!1UP
Thank you @luizeba for the rating and support.
You have received a 1UP from @luizeba!
@stem-curator, @neoxag-curator
And they will bring !PIZZA 🍕, !PGM 🎮 and !LOLZ 🤣 The @oneup-cartel will soon upvote you with:
Learn more about our delegation service to earn daily rewards. Join the Cartel on Discord.
Thanks @curation-cartel for the input on my work.
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
You may also include @stemsocial as a beneficiary of the rewards of this post to get a stronger support.
Again grateful to you @stemsocial with each support and valuation that you make on our articles, motivate us to continue sharing quality material.
Success in your work, be well.
I enjoyed reading this blog, and I would actually like to raise a small comment (if I may) which does not change anything relative to what you wrote.
There is in fact more than three states of matter, although the extra ones are not typical ones. The fourth state of matter is plasma, and we must then add to the list a bunch of more exotic states like supercritical fluid, degenerate matter, etc. A quite complete list can be found here.
Cheers!