Greetings and welcome dear constituents, continuing with the approach of scientific content, this time we will be sharing information associated with organic reactions and their impact on the preparation of explosives and as has been constant throughout my publications, this type of material is usually shared through the @Stemsocial community, a leader in the promotion and dissemination of the scientific area within the #Hive platform.

Image courtesy of: thomasstaub
In this sense, it should be noted that when we start the scientific approach associated with organic chemistry, we enter a kind of mental gap because we find ourselves with a maddening set of millions of compounds and dozens of functional groups that work with each other and have different physical and chemical properties, not to mention the chemical behavior that these assume when they are part of a chemical reaction.
However, when we begin to detail each process, we realize that these are not performed in isolation and organic chemistry is developed under logical questions, hence it has been organized according to families and functional groups and work according to the properties and characteristics presented by these, which has allowed substantial progress in the development of this area of chemistry.
Consequently, at the organic level, chemical processes are carried out in four specific reactions of which we can highlight the addition reactions which represent a process of combination between two reactants with the purpose of forming a product different from the previous ones, an example of this can be obtained when an alkene is subjected to a halogenation process which results in an alkyl halide.
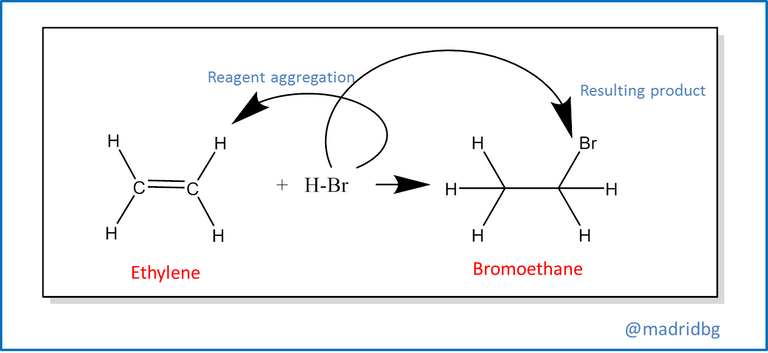
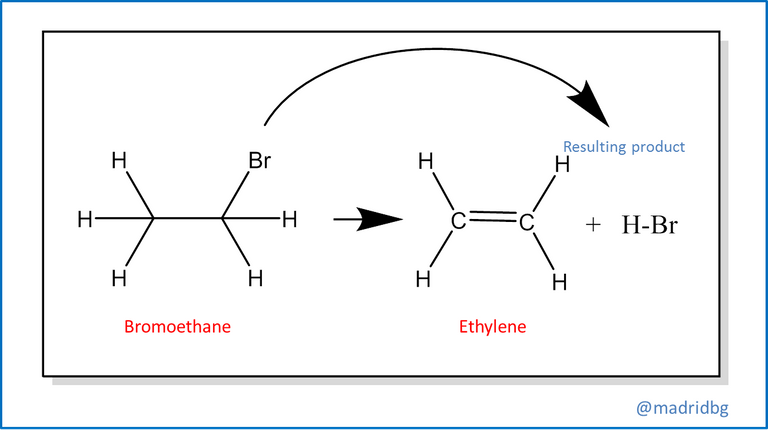
Also, we find the elimination reactions at this point usually occurs the opposite of the above, so that from a single reagent we run a decomposition of the same and allows us to obtain 2 products with different properties to the reagent that conforms it, among this reaction we can highlight the process of decomposition of bromoethane, which in alkaline medium is capable of forming ethylene and failing that the respective hydrobromic acid.
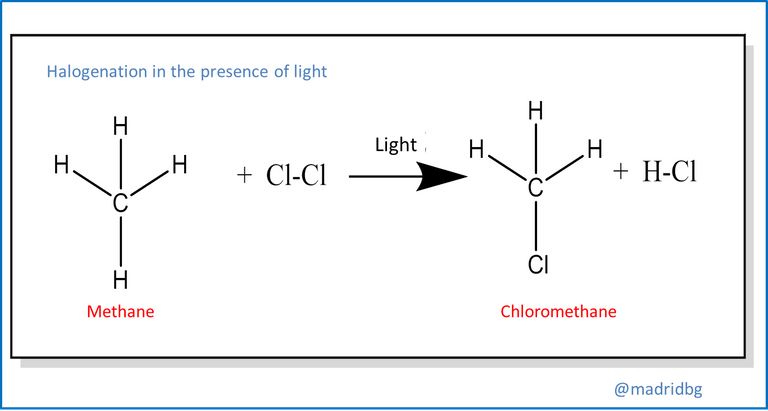
On the other hand, substitution reactions usually occur when two reactants are able to exchange their parts and originate new products, to exemplify this process just study the reactions of any alkane in the presence of chlorine gas and ultraviolet light, the result of this mechanism is an alkyl chloride and its respective hydrochloric acid, which starts through a process of substitution of a hydrogen atom of the respective alkane.
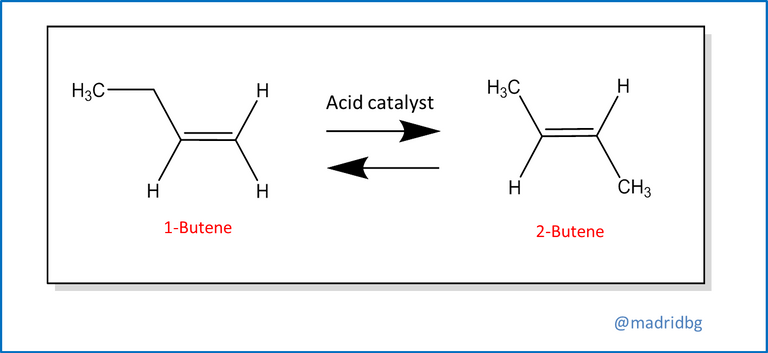
Last but not least, we can find the rearrangement or transposition reactions, which are originated when a single reactant undergoes a rearrangement of the bonds or atoms that constitute it, to give rise to a product with isomeric characteristics, An example that we can visualize through the transposition or conversion of 1-butene in its respective isomer 2-butene, note that from this chemical reaction the amount of atoms present is not altered, but through the acid catalyst that is immersed in the process, the double bond is usually moved from the position it had initially.
Now, if we analyze the four previous processes we can think that they are, if you will, easy to execute, so from this it is valid to ask ourselves how do organic reactions happen? And to answer this question it is necessary to address the reaction mechanism that is immersed in them, since from this, we can describe in detail exactly what happens at each stage of a chemical transformation, i.e. we can predict what type and which bonds are broken and what is the respective order of this rupture, also how many and which bonds are formed and what are the relative speeds of reaction that occurs in each of the stages.
Consequently, a chemical reaction at the organic level is a complex process that involves breaking and forming bonds when molecules are joined together or when molecules are separated, so that at the energy level when a bond is formed, heat absorption is generated in the process (endothermic system), Otherwise, when the bonds are broken, what is produced is a release of energy in the form of heat (exothermic process), a mechanism known as bond dissociation, which is defined as the amount of energy necessary to break or produce radical fragments when the molecule is in the gas phase.
Now, starting from the above theoretical constructs, let us analyze how reactions develop in the synthesis of explosives, knowing that most chemical reactions are carried out in one or more discrete stages and each of them has a reaction rate, an equilibrium mechanism and a well-defined constant or process.
However, although the variables are known and controlled, at the level of explosives this theoretical process is complicated, since the explosions are different, their velocities are so fast and their mechanisms so complex that the details of how they occur completely defies logical interpretation from a scientific point of view, What is possible to know is that in the middle of the chemical explosions a process of decomposition of the molecules into fragments is generated, which immediately recombine to form the final products, which generally tend to be stable gases such as nitrogen, carbon dioxide and, failing that, water vapor.
At this point, the result of the almost instantaneous release of large amounts of the gases mentioned above, generate a kind of shock wave with an imminent and devastating force to expand, so that this shock wave is capable of traveling at speeds above 9000 m / sec, equivalent to 20000 min / hour, thus generating pressures of more than 700,000 atmosphere, no doubt an impact of this magnitude implies a devastating force in the spaces or surroundings where they run.
At a chemical level, the process of explosives can be classified in two, primary explosives that depend on their sensitivity to shock or percussion, among these compounds we can find lead azide as one of the most sensitive and are used in detonators and military fulminants. On the other hand, secondary explosives, also known as high explosives, are less sensitive to heat and percussion so that their manufacture and transportation are much safer and this is due to the fact that most secondary explosives simply burn and do not explode when ignited in the air, hence most of these explosives usually detonate due to nearby explosions or because of a primary initiator.

Image courtesy of: Loren Elkin
Among the main explosives at the secondary level we can find nitroglycerin, which was prepared in 1847 from a reaction of pure glycerin with nitric acid in the presence of sulfuric acid and as expected the reaction occurs at an extremely fast and dangerous rate.
So different methods have been implemented to safely contain the displacement of the nitroglycerin reaction and consequently industrial dynamite has been developed, which is used to exploit quarries and open roads and this is a mixture of ammonium nitrate and nitroglycerin absorbed in diatomaceous earth, which provides greater stability and reaction control during the process.
If we approach a little more in detail the mechanism of explosives, we can realize that within the military component the explosives that are normally used are bomb fillers and these must be sensitive to impact when they explode and have good stability to be able to store them for long periods of time, among these we can highlight the trinitrotoluene, pentaerythrol tetranitrate, we can also find mixtures with waxes and synthetic polymers for the manufacture of plastic explosives.
Consequently, this is an area that has been studied in depth since it represents an apparent security mechanism for the States and has been developed through scientific advances.
BIBLIOGRAPHY CONSULTED
[1] Rincón Flórez, J. F.; Fonseca Becerra, J. E. & Carvajal Medina, R. J Calculation of thermodynamic parameters for military explosives. Application of thermodynamic fundamentals and properties of military explosives.Artículo: Acceso Online
[2] Chang, R. (2010). Química. Decima edicion. McGraw-hill Interamericana editores. ISBN: 978-607-15-0307-7.
[3] Ralph, H. Petrucci, William S. Harwood, E. Geoffrey Herring. (2003). QUIMICA GENERAL. Octava edición. PEARSON EDUCACIÓN. S.A., Madrid.
[4] WADE,LEROY. (2011). . ORGANIC CHEMISTRY. VOLUME 2. SEVENTH EDITION. PEARSON EDUCATION, MEXICO, 2011 ISBN: 978-607-32.()793-5.
OF INTEREST
1. The molecular models presented were designed by @madridbg using Chem3D and Chemdraw software.
2. For more information related to the areas of science, technology, engineering and mathematics, do not hesitate to visit #stemsocial and #stem-espanol, they are communities that promote scientific advances in these areas

The rewards earned on this comment will go directly to the people( @madridbg ) sharing the post on Twitter as long as they are registered with @poshtoken. Sign up at https://hiveposh.com.
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
Thanks for including @stemsocial as a beneficiary, which gives you stronger support.
Su post ha sido valorado por @ramonycajal
I remember my organic chemistry teacher talking about that
!1UP
You have received a 1UP from @gwajnberg!
@stem-curator, @neoxag-curator, @pal-curator
And they will bring !PIZZA 🍕. The @oneup-cartel will soon upvote you with:
Learn more about our delegation service to earn daily rewards. Join the Cartel on Discord.
I gifted $PIZZA slices here:
(3/20) @curation-cartel tipped @madridbg (x1)
Learn more at https://hive.pizza!