Who can forget their jolly childhood days? We played with our friends in the playground, and we ran all around the house. What a time it was. However, sometimes while playing, we suffered injuries and wounds. Of course, those wounds healed, but then some wounds left scars. You would still find these scars where the damage was 30 years ago. But the good news is that it stopped scarring. Imagine a disease in which this process of forming a scar never ends. The hard tissue that is the scar keeps growing beyond the margins of the wound and forms a solid lump. Indeed, this is what happens in a fibrotic disease of the skin called keloids 1, 2. This excessive scarring of the tissue leads to tissue hardening and hence loss of function and it is termed fibrosis 3.
All organs scar and develop fibrosis in response to an injury
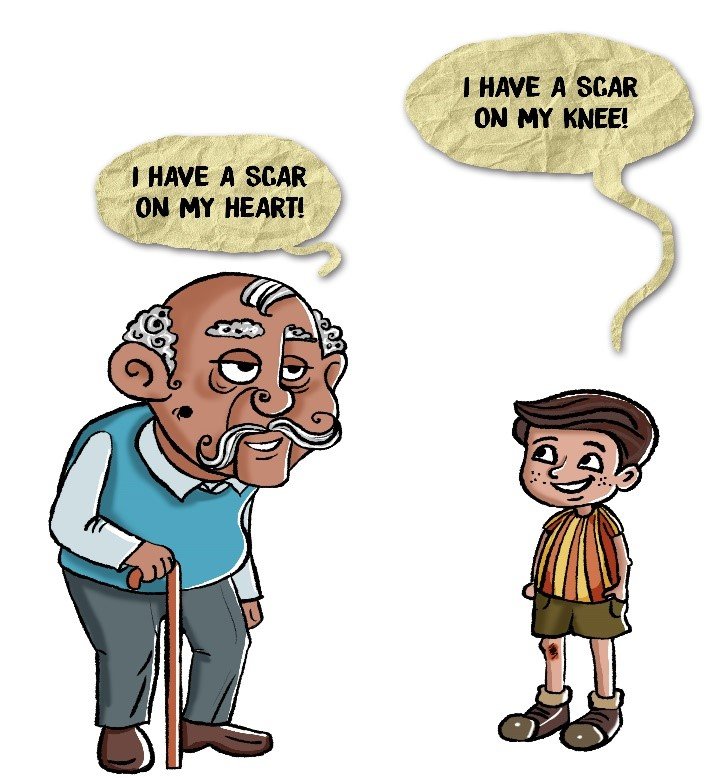
Nevertheless, the skin is not the only organ that gets injured and scarred. For instance, after a heart attack, the heart tissue suffers damage because some cells die due to a lack of oxygen. It starts the healing process and forms a scar in the heart 4. The hardening of the heart tissue hampers its functioning, weakens the heart and makes you prone to cardiac arrest and death. High glucose levels in the blood also damage tissues owing to glycemic injury. This is the major cause of cardiovascular disease, diabetic nephropathy (renal fibrosis), and other secondary ailments in diabetic patients 5. Sometimes, we injure our own organs due to lifestyle choices. For example, when we smoke, we wound our lungs and put ourselves at risk of developing lung fibrosis 6. When we drink too much, we harm our liver which is the cause of liver fibrosis (liver cirrhosis) 7. Lifestyle choices and high blood pressure (hypertension) can amplify the usual wear and tear that happens in arteries. The atrial damage causes fibrotic plaques to form inside the blood vessels, a disease commonly known as atherosclerosis 8. Then there are some disease-causing germs which cause injury to organs. Take Covid-19 as an example. People who suffer from severe Covid-19 suffer massive damage to their lungs. Hence, one of the concerns for severe Covid-19 patients even after their recovery is the development of lung fibrosis which can cause long-term lung dysfunction 9, 10.
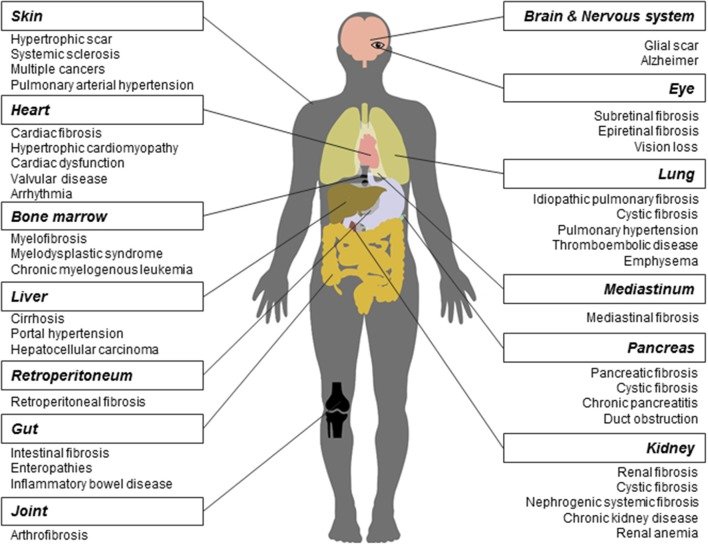
Reproduced from Li et al., 2017. Front. Pharmacol. 8:855.11. CC BY 4.0
However, it does not always have to be a heart attack, a pathogen, a lifestyle choice, or a lifestyle disease like diabetes that initiates fibrosis. Sometimes, it can start because there are dysregulated molecular signals that mimic the wound-healing response, without having any actual wound, anywhere. Or it can happen due to an autoimmune disorder, in which immune cells attack and damage the body’s own tissues. An example of such a fibrotic disease is scleroderma (also known as systemic sclerosis) 12, where the fibrosis starts in the skin in the extremities of the limbs(fingers and toes) and then spreads to other organs – lungs, heart, and kidneys.
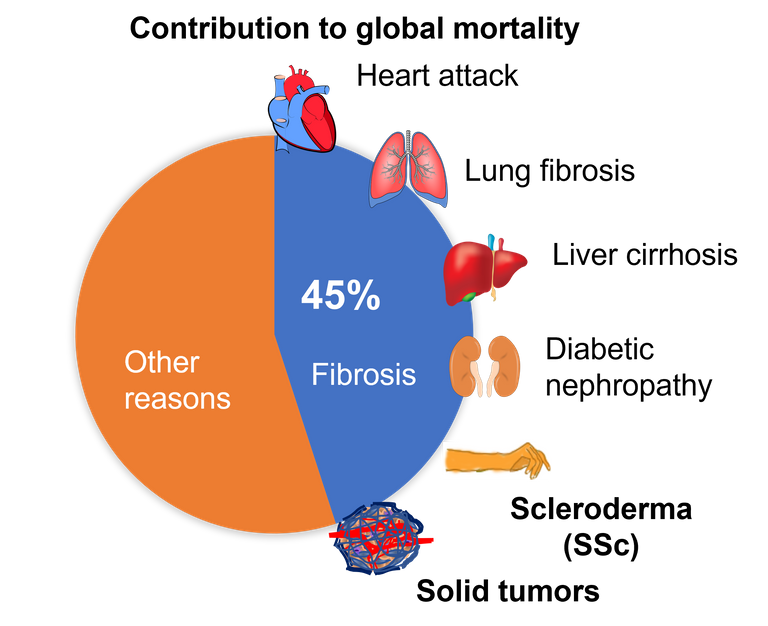
Overall, fibrosis is either a cause or a consequence of many chronic diseases. Even, the tissue surrounding the cancerous tumour, known as tumour stroma, is a hard-fibrotic tissue, which nurtures the tumour and helps cancer to spread 13, 14. Overall, fibrosis is the cause of 45% of deaths that occur worldwide 3. And yet, we have no cure for fibrosis. However, if we try to understand how a wound heals and what goes wrong that sometimes the scarring does not stop, then we can come up with solutions.
A brief look into the wound-healing.
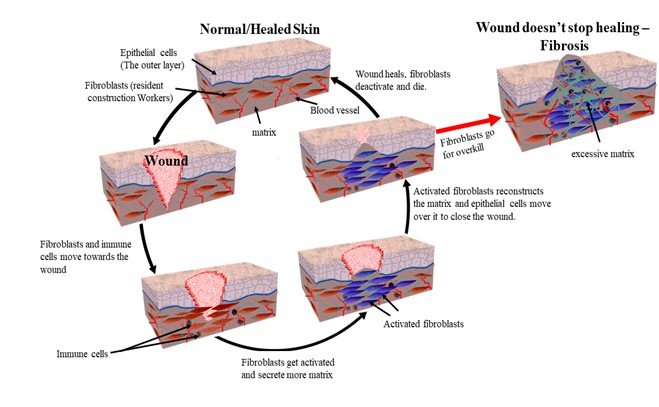
Let us take an example of a skin wound. As soon as you cut your skin, the first response of your body is to limit the blood flow. The blood vessels constrict, and the clotting factors in the blood get activated and form a clot. A few hours later, the blood vessels dilate, allowing immune cells like neutrophils and macrophages to enter the wound site. Their job is to clear up the damaged cells and fight any invading bacteria that might have entered the wound. Then comes the resident construction workers of the skin – fibroblasts – who live in the bottom layer of the skin called the dermis. Their typical job is to make the matrix for the tissue. The tissue matrix is like cement that holds the tissue together. When they see a wounded tissue around them, they become hyperactive in producing and secreting this matrix 15.
The epithelial cells which form the top layer of the skin visible to us then migrate over this matrix to close the wound. Meanwhile, the fibroblasts continue to organise the matrix secreted by them. They aim to restore the architecture of the tissue. After restoration, the fibroblasts deactivate, or they die. This organisation of the matrix can go on for years. And this is what that 30-year-old scar you have is all about. However, in some cases, these construction workers go for overkill. That is, they remain hyperactive and keep producing the matrix, leading to the development of fibrotic disease 3.
The healing process is similar in all organs across the body and shares common cellular and molecular pathways. And at the centre of all fibrotic diseases are these construction workers – the fibroblasts. The key here is to understand the nature of these construction workers and why they stay constitutively active in fibrotic diseases. Are there false signals that are telling the fibroblasts to remain active? Are there dysregulated immune cells causing more damage to the tissue before it can heal? Or is there something intrinsically wrong with these fibroblasts? In order to answer these questions we needed a mouse model which can mimic a human fibrotic disease.
Creating a disease model for human fibrotic skin disease scleroderma.
We know that the foetal wounds do not scar, but adult wounds do 16. The mouse skin repair perfectly, but human skin form scars. We noticed that there is a protein called Snail which expressed in skin epithelial cells of adult humans when they are wounded, but this does not happen in the mouse. Moreover, we found that this protein is expressed in skin epithelial cells of patients with skin fibrosis (scleroderma), but not in healthy people. So, we created a mouse via genetic manipulation, such that it constitutively expresses this protein in the epithelial cells as human skin fibrosis patient do? Will they scar if we wound them? They do, just as we predicted. But what was more interesting is that they scarred all over the body even without a wound. These mice displayed all the known symptoms of human fibrotic skin disease - scleroderma. The fibrosis started developing in the skin of extremities (tail and limbs), and it spread throughout the body and internal organs and these mice as they aged.
Using this mouse model we found interesting targets for for developing novel therupatics for scleroderma. But more on that later. I will describe our findings in more details in part 2. Meanwhile, if you are interested to read it you can read our recently published paper in JID in which I am a co-first authror.
Paper on which the blog is based

Hive#STEMsocial powered by
Video by @gtg
References
About contacting me
You can reach out to me on discord if you are in stemsocial discord. Or you can even send me a DM. My discord handle is the same as my hive - @scienceblocks. You can also ping me on my telegram handle: @UncertainHeisenberg. Or follow me on Twitter: @scienceblocks1
I have added @mycrimsonhues and @gtg as 1% beneficaries as token of thanks for helping with the illustrations and stemsocial video used in this post, respectively.
That's an original way of looking at the top cause of death! We're used to it being heart disease, but you're saying the ultimate cause of death behind heart disease and other stuff could be attributed to scarring. I would say maybe it's aging? Overscarring doesn't seem to be present in very young people? And most diseases seem to happen after a certain age.
Well in a way you can say that ageing is indeed the cause of most deaths. One of popular theory of ageing however is accumulation of molecular, cellular and tissue damage. Repairing the damage is of utmost importance at any level. Both failing to repair the damage and going on an overdrive can prove detrimental. At molecular level the damage is mutations accumulated in somatic cells with age. And DNA repair pathways often try to fix them. But take example of choosing to repair vs choosing to initiate senescence or apoptosis. There needs to be fine balance maintained between these pathways. Overdrive of Non-homologus end joining can cause further DNA damage, excess of senescence however may induce senescence associated secreted proteins (SASPs) which increase inflammation and promote tissue damage.
I like the example of cardiovascular diseases because it exemplifies both importance if ageing and fibrosis. First myocardial infarction (heart attack) maybe induced by underlying atherosclerosis (associated with vascular fibrosis) or due to injury to heart cells due to glycemic injury (in diabetics) or underlying low-grade inflammation owing to ageing and stress. The lack of oxygen can then cause ischemic injury, which the heart would try to repair after the recovery from the attack. The healing process often leads to a scar, which hinders proper functioning of the heart. The second best example is of solid tumors, a mass of cancerous cells (often more likely in old age) induce a desmoplastic reaction, causing fibrosis, which hinders function of the tissue where the tumour is. On the other hand, fibrotic tissues are highly prone to development of cancers. In fact prostate cancers before spreading to bone remodels the bone matrix before establishing secondary site of the tumor. So while age can be attributed as the most obvious cause of death, the tissue hardening due to accumulated damage or due to age associated disease is what causes tissue dysfunction. Fibrosis is a condition or state of tissue rather than being a disease itself.
Now of course there are deaths that happen which doesn't involve fibrosis (about 55%) but other tissue conditions. These include failure to repair damage (chronic wounds for example), traumatic injuries (accidents and suicides), cytokine storm, sepsis, haemorrhages or other acute failures.
Thorough response!
Is it correct to say that our body/genes don't cause aging? I mean, there's no gene for aging, let's say. Aging happens because the mechanisms that keep us alive are imperfect, and because of evolutionary reasons (say, it serves the genes better to switch to another body through procreation rather than maintain a very old one). Is that correct to say? Or is there some truth to the idea that "we are programmed to age and die"?
Yes it would be correct to say that our body/genes don't cause ageing. Although our genes do try to delay it. These are genes associated with repair and genes regulating them. Mutations in these genes is associated with accelerated ageing and early onset of old age-associated diseases. These are genes involved in management of DNA damage repair, cellular senescence, inflammation and stem cell maintenance. At cellular and tissue level body has stem cells which is like a reservoir for all cells in that tissue. Stem cells provides precursor cells for all other cells in the tissue. This keeps the tissue young and healthy. Nevertheless, as we age then due to loss of stem cells or due to loss of their ability to differentiate (or lack of signals that activate stem cells or presence of inhibitory signalling) the tissue loses its ability to rejuvenate. An example of this is loss of melanocyte stem cell and/or their ability to differentiate leads to greying of hair.
If you look at the bigger picture, one of the major property of life is to keep the system away from equilibrium. Each cell and molecule in the body attempts to reach an equilibrium state and maximize the entropy. But active process in the body oppose these. But the nature eventually wins. With wear and tear accumulating over time these active processes malfunction. And when the equilibrium is reached cells and eventually we die.
Another way to look at ageing is loss of information. There is information in DNA for instance which is actively maintained by cells. However, cells lose their ability with age to maintain this information. Recent studies show that recording this information can reverse ageing.
So your second statement is also true. The mechanisms to avoid ageing are there, but they are not full-proof. Understanding these mechanism will help us slow down ageing (or even reverse it, hopefully).
I don't think that the third statement if true. We are not programmed to age and die. That's just law of nature (a default program) that everything just maximizes entropy and moves towards equilibrium. Life processes are about staying away from equilibrium and decreasing local entropy. Our genes/cells/and tissues tries their best to fight the nature. Nevertheless, the nature eventually wins.
Yes I once described life as anti-reality! Sometimes I even describe life as anti-life! In the sense that biological life basically opposes 'life' meaning reality, or nature, or entropy. But if, as physicists are telling us, entropy is a sure thing, then life is doomed to fail. So we're basically fighting a hopeless fight! I don't mean to sound nihilistic, cause I'm definitely anti-nihilist, but it's just an interesting way of looking at things.
The way I view biological bodies, is that we're already immortal, just not at the level we want. We are immortal at the gene level, not the person level. The genes use the body as a vehicle, and discard it as they see fit. But an old person can still produce a baby with young cheeks, young heart, free of cancer, etc., so in principle the ability to fix everything is there, never dying.
Loss of information is a rule of nature, like if you keep xeroxing a piece of printed A4 paper it will eventually just become a blank page or full of nonsense letters. But the constant pressure of natural selection gets rid of mistakes so the genes copying themselves don't get progressively worse at it. Trying their best to fight nature, as you say!
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
Thanks for including @stemsocial as a beneficiary, which gives you stronger support.
Very interesting! thats whay you wrote about SNAIL previously!
!1UP
I mean it was a different study. Snail Transgenic mouse is a dual model for both cancer development and fibrosis. And I worked on both aspects. I am definitely going to draw a connection between these studies in future blogs.
You have received a 1UP from @gwajnberg!
@stem-curator, @vyb-curator, @pob-curator
And they will bring !PIZZA 🍕. The @oneup-cartel will soon upvote you with:
Learn more about our delegation service to earn daily rewards. Join the Cartel on Discord.
I gifted $PIZZA slices here:
(15/20) @curation-cartel tipped @scienceblocks (x1)
Join us in Discord!