So the thing is the current Covid19 crisis has kept some of us really on our toes at work. Also, since Sars-CoV-2 is all we are allowed to work on for now, that is all I have been reading. So I thought why not share some of the papers that have been running through my head. So here is the 1st journal club on this topic.
.jpeg)
why they don't fall sick!
Source | pixabay
Given, the current Coronavirus crisis, we must have all heard a lot about bats as of now. One of the things that you must have heard that Bats are reservoir to many zoonotic viruses. Even the current virus that has caused the crisis SARS-CoV-2 shows 96% similarity to a bat coronavirus RaTG13 (Zohu et al., 2020). It is true that bats do harbour a lot more zoonotic viruses, than any other known mammal (Olival et al., 2017). But, how is it that bats don't keep getting sick and falling dead on the ground?
Well, this is exactly what this paper published by Ahn et al., 2019 talks about. How bats carry this virus and still don't fall sick? Can lessons from bats help in reducing Covid19 related deaths, or for that matter other viral related deaths in humans?
The idea of the paper revolves around a specific kind of immune response that is heightened in humans but dampened in bats. And, since the excessive immune response is what causes many of the symptoms and even mortality in many infectious diseases; it might be possible to carry the virus safely by dampening some of the more violent immune responses.
The NLRP3 Inflammasome
To be more specific, the NLRP3 Inflammasome response is what we are talking about. NLRP3 is a protein in cells, which gets expressed when another protein NfKb is activated due to a stress signal - when molecules from damaged cells or external pathogens bind to toll-like receptor (TLR) on certain immune cells. Once there is enough NLRP3 in the cells, another stress signal opens up potassium ion channels which in turn causes a change in confirmation of NLRP3 protein. This activated form of NLRP3 then forms megastructures by aggregating together. Another protein called ASC binds to this mega structure created by NLRP3 aggregation and forms ASC filaments. These aggregated filamentous structures can be seen as specs inside the cells. The ASC filaments then recruit a protein called Caspase1 via its card domain. A lot of caspase1 molecules in close proximity cleave each other exposing their catalytic domain.
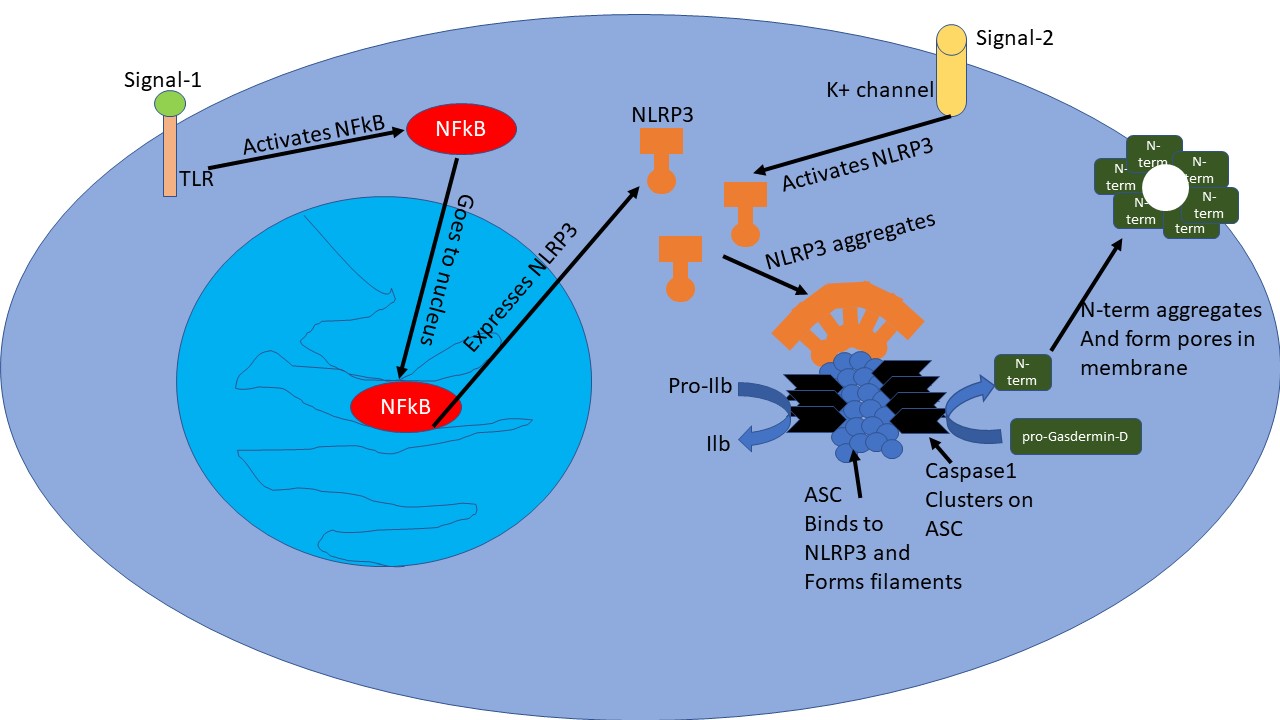
Illustrated by @scienceblocks
This active caspase1 is now ready to cleave other proteins - it cleaves pro-Il1b and pro-Il18 to yield Il1b and Il18 inflammatory cytokines. Caspase1 also cleaves pro-Gasdermin-D. The cleavage of Gasdermin-D releases its N-Terminal region, which then aggregates in cell membrane creating pores that eventually kills the cells. The cell leaks out inflammatory cytokines Il1b and Il18 into the surrounding tissue along with other cellular contents. While cellular contents may act as a signal for another nearby cell to induce inflammation, the inflammatory cytokines recruit immune cells at the site.
Here is a link to a nice animation of NLRP3 inflammasome
The story
Ahn et al. took peripheral blood mononuclear cells (PMBCs) - lymphocytes (T-cells and B-cells) and monocytes (precursors to macrophages) from human bat blood. As a first signal, they primed the cells with lipopolysaccharide, a component of the bacterial membrane. Then as a second signal, either via ATP (extracellular ATP signals cellular damage) or via Nigericin a toxin and an antibiotic derived from Streptomyces hygroscopicus - they activated the NLRP3. So ATP acts as if it is a signal from the body, while nigercin act as a signal from the pathogen. Nevertheless, in both cases, bat PMBCs showed a lower amount of ASC specs and reduced IL1b secretion as compared to human PMBCs. The authors also compared the response of macrophages and dendritic cells derived from the bone marrow of either bats or mice. And yet again looks like mice cells had a stronger inflammasome response as compared to bat cells.
So what the hell is going on here? Why is it that bat cells don't show such high inflammasome activity? Is there a problem with NFkB activation to begin with? Turns out, other genes such as Il6 and TNFa under NFkB regulation just do fine. Nevertheless, expression of NLRP3 was lower in bat cells as compared to human or mouse cells. They tried multiple signals known to activate NFkB pathway - bacterial, cellular damage and even analogue of viral DNA. But nada, NLRP3 expression was weak in all cases.
On top of that they found that when it comes to binding to ASC protein, the human and mouse NLRP3 was much more potent than bat NLRP3. Moreover, in bats, NLRP3 comes in 4 flavours due to alternative splicing - a process by which cell create multiple flavours of proteins from the same transcript of gene - by removing different portions from the gene transcript for a different flavour. Of these 2 flavours lacked a sequence of a gene - exon 7 - and was even worse than the other 2 flavours when it came to binding to ASC.
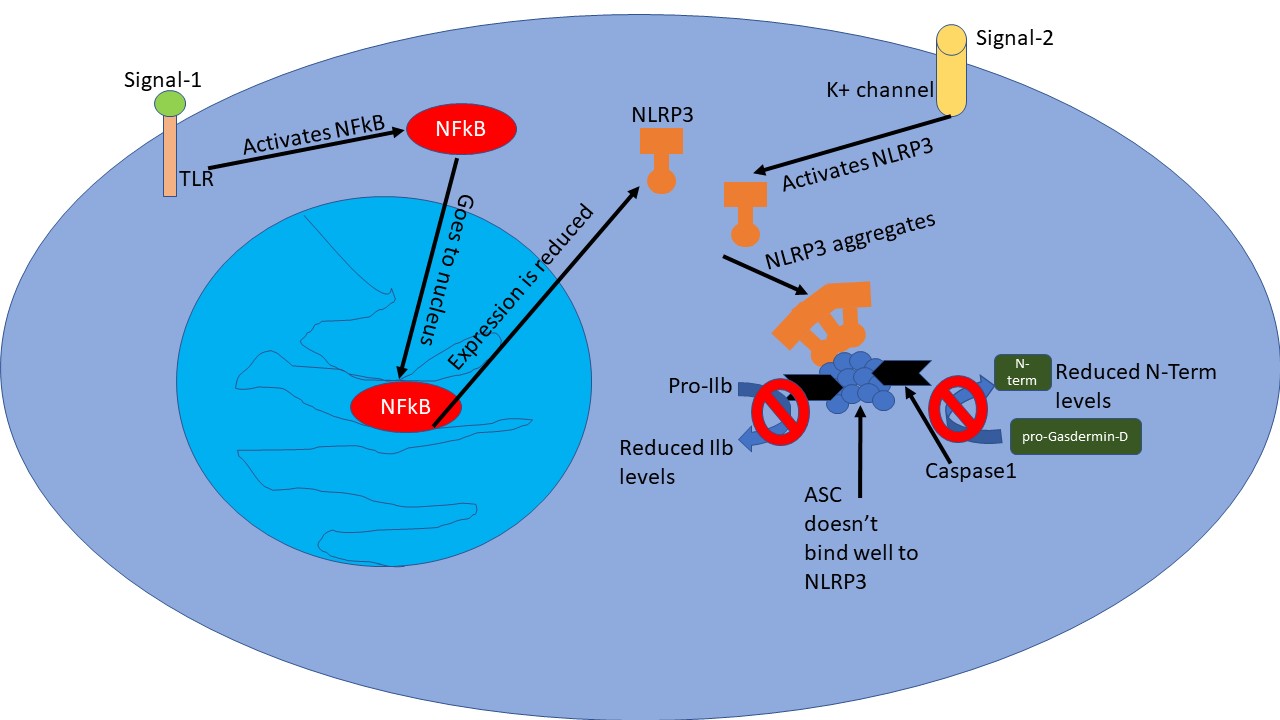
Illustrated by @scienceblocks
They finally tested how different viruses - Influenza A, PRV3M , and MERS coronavirus affect bat cells compared to humans or mouse. It turns out that these viruses don't activate NLRP3 inflammasome in bat cells.
Well, at least from these in vitro cell culture studies it looks as if bats cells are not oversensitive to the virus, at least when it comes to NLRP3 inflammasome mediated inflammation. It is likely that because of dampened NLRP3 inflammasome response bats don't become as sick from these viruses as other mammals do. I mean even Ebola don't fucking make them sick nor does the other coronaviruses. And it's not as if they eliminate the virus very fast, they carry it around in high titer without falling sick (Swanepoel et al., 1996, Munster et al., 2016). The question, however, is why did bats evolve such an ability? Like did they really evolve to be viral reservoirs? Or being a viral reservoir is a byproduct of something else?
You must have noted in our discussion that the NLRP3-Inflammasome response is dampened in bats not only to pathogenic signals but also to cellular damage signals. Now bats are the only flying mammals. Which means their metabolic rate is pretty high. And, a high metabolic rate implies a lot of cellular wear and tear because of reactive oxygen species generated during cellular respiration, for example. If the immune cells were oversensitive to every cellular damage then they would have wreaked the havoc inside the bats. So its likely bats evolved NLRP3 pathway in a way that its immune cells don't overreact to metabolic damage. And, becoming a virus reservoir was just a side effect.
Also, if NLRP3-inflammasome is what is causing the symptoms and damage in humans when it comes to human viral diseases, esp the current crisis of Covid19, will dampen this help? I mean it's worth a shot to try it on animal models at least for now.
Nevertheless, there are a couple of things I think authors could have done more is - one, they should have tested their hypothesis in-vivo. It would be ideal to make a transgenic bat with human NLRP3 gene and test this hypothesis, but they could have tried it in mouse model at least. What if we make a transgenic mouse with bat NLRP3? Would it be able to tolerate more viral load without getting sick? Will NLRP3 pathway inhibitors reduce severe symptomatic viral infections in mice and to what extent?
The second thing I wish authors did was to find why NFkB doesn't cause high expression of NLRP3 in bat cells? Is the sequence of NLRP3 promoter different or is there a mutation in bat NfKb?
Well that's it for today.
About STEMSocial
But, before I go I would like to mention about the STEMSocial platform. Well, if you love reading and writing interesting science articles @steemstem is a community on hive that support authors and content creators in the STEM field. If you wish to support steemstem do see the links below.
Or delegate to @steemstem.
Also, if you have any questions regarding steemstem, do join the steemstem discord server.
You can DM me on discord, I have the same handle - @scienceblocks. Also if you are not a steem user, and reading this blog inspired you to start your science blog, find me on discord and let me know about you. I can try and help you navigate your way through steem.

References
Zhou et al., 2020. A pneumonia outbreak associated with a new coronavirus of probable bat origin
Olival et al., 2017. Host and viral traits predict zoonotic spillover from mammals
I read this blog a couple of times with great interest. The balance between inflammation and infection is one that has interested me for some time. Some years ago I began a protocol of daily, low-dose prednisone (3 mgs). I was very aware that it was kind of a devil's bargain, but sometimes that's the only bargain in town.
I'm familiar with the 'cytokine storm' that, paradoxically, is treated in severe flu with immune suppressive drugs--which also suppress inflammation. When I 'catch something' often my doctor will use a double-pronged approach: up the dose of prednisone and back it up with an antibiotic to fight secondary infection.
Here's the articleHi @scienceblocks, After reading your response to @gentleshaid I did some poking around to get COVID-relevant information on the tension between inflammation and infection. The article is 'old' (anything published before yesterday is old in this environment), published on March 25, 2020. However I do think the authors treat the inflammation/infection paradox well, in relation to COVID. Bottom line, I think: every patient is unique and medicine is an art. There are few fast rules. The smartest course for a patient is to pick a clever doctor who cares. And then, roll the dice :))
As usual, I am impressed with your knowledge, your ability to share it, and most of the sense of humanity that infuses everything you write.
Very well articulated. And it is very important for letting the doctors choose if and when the anti inflammatory drugs should be given. And a good doctor who cares and can make that choice is very crucial.
There are two regions of the coronavirus orf3a which is known to activate NLRP3 response and orf9b which inhibits anti-viral interferon response. I think drugs against those might come handy to more specifically tune the immune response. Lets see what comes out with time.
Thank you for that gracious response. I'm going to read up on orf3a, NLRP3, and orf9b. It's likely I'll find the material challenging, but I am curious to see what sense I might make of it.
Good health to you and your family
I will try to address those questions in this common reply.
I think I need to add a little more perspective into it. Every time, there is an external pathogen or even internal damage the immune system needs to respond. What we have here is too strong an immune response or too weak an immune response or just right immune response.@gentleshaid @enforcer48
Think of this way. When you get a wound on your skin, immune cells arrive there to clear up the mess and stop pathogens from entering. But this response is regulated by various brakes. After a while, these immune cells have to deactivate or die and stop messing around. If they don't you end up getting chronic wounds which never heals because the inflammation in the wound keeps damaging the tissue further. However, if there was no inflammation some microbes will digest and eat your tissue like it was a piece of bread.
Another example is the fever. Fever is caused by a signal generated by immune cells with effects the thermostat in your brain. This causes the temperature to increase and make the environment unsuitable for the pathogens to thrive. But after a certain point, too much temperature say above 104 degrees or having temperature for a long time can damage your own organs. So up to certain point fever might aid fighting germs but after that its self-damaging.
Same goes for cough. A reflex generated by the interaction between immune cells and neurons during a respiratory infection.
Then coming to bats - it is true that certain kind of immune responses to viruses is dampened in bats. In this paper, it is NLRP3 inflammasome response. Another example would be dampened response of STING pathway in bats to damaged DNA or DNA virus in the cells. But it's not completely true that bats don't do anything to keep the viral titers in check. For instance, some studies suggest that interferon response is constitutively active in certain bats esp for interferon A. Even in this paper you will notice that while NLRP3 expression was dampened in bat cells, other inflammatory genes under NFkB regulation were not as low.
Which brings us to the next question? Would anti-inflammatory drugs work? The thing again is you don't want to dampen your immune system blindly. It is required to do slow viral replication, clear up damaged tissue and even help in developing antibody and T cell-mediated immunity against the virus. IMO, what one may want to target are more specific pathways that causes much violent inflammation. For instance, this paper suggests testing NLRP3 inhibitors might be interesting. So it totally depends on the mechanism of action of anti-inflammatory in question.
Anyhow, thought would share some interesting links for further reading -
How flu kills people
Going to Bat for Studies of Disease Tolerance
Brief, educative, and interesting to read. I never knew that excessive immune response is what to leads to symptoms in humans. Is this really that in all cases?
If such is the case, why then do we point accusing fingers to the immune system when diseases take over talk about 'low immunity'? Does it mean that low immunity is equivalent to an excessive immune response?
Good question!
You deliver us this incredible info about how bats just fly around as “viral reservoirs” and end your post with :Well, that’s it for today! 😆 I loved it! It was also interesting to learn that that specific inflammatory response (or lack of) in bats is similar in both cases of pathogenic and cellular damage signals.
I’m now wondering what on earth makes bats sick!? I’m going to google that right now! :)
You take care :)It’s always great to read your work @scienceblocks!
Do you mind sharing the answer with me? I am too lazy for googling this, and this is an interesting question!
It's curious right? I had to persist on my search as, it appeared to me, the focus of what is out there about bats and diseases is on the fact that they are massive pathogens reservoirs. But then I found something which made for an interesting reading Ref..
In a nutshell some of the causes of death in urban European bats were found to be:
An interesting observation is that it appears that bats are more likely to succumb to (bacterial and viral) infections when they are already in a state of debilitation (e.g. due to injury).
So, there you go!
I wish you & the family a very good day 😊
Thanks for sharing this information with me. I may have a look to the reference later today. For now, I am writing on dilaton dark matter (future research paper; all results are there but we need to put that in words)...
Happy writing 😊
Brief, educative, and interesting to read. I never knew that excessive immune response is what to leads to symptoms in humans. Is this really that in all cases?
If such is the case, why then do we point accusing fingers to the immune system when diseases take over talk about 'low immunity'? Does it mean that low immunity is equivalent to an excessive immune response?
That bat has nice balls
Lol. I think after knowing this one would need even bigger balls to consume uncooked bats though! 😂
Damned! You see balls everywhere!
@tipu curate
Upvoted 👌 (Mana: 24/40)
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider supporting our funding proposal, approving our witness (@stem.witness) or delegating to the @steemstem account (for some ROI).
Thanks for using the STEMsocial app
and including @steemstem as a beneficiaryi, which give you stronger support.
Wow! I knew bats were reservoirs to many viruses, but I didn’t know that the Ebola was just a joke for them. Crazy bats. Any realchance we could get some information in a close future to help us with the covid from there?
Well there is this idea that if we understand how bats tolerate the coronavirus we can try to mimic the same with drugs. The Orf3a of SARS and SARS2 (covid19) has been implicated in activating nlrp3 pathway for instance.So one of the strategies could be to target interaction og orf3a with the human protein it binds to, or drugs that mellow down nlrp3. I am thinking of writing a grant around this or at least include it in another grant that I am already writing. Let's see.
Oh, my fingers are crossed for your grant proposals, so that I could eventually get the answers to my questions ;)
I read that about COVID-19 as well. It makes you wonder if taking anti-inflammatory drugs is the way to go should the vaccine fails.
Do anti-inflammatory drugs mitigate excessive immune response?
It should.
I mean, they make transplant patients take them. I don't know the specifics since I've never studied into those, but the principles make sense.