Hi hivers, hope you are all doing good?
Do you know that fuel, plastics, rubber, cosmetics and so on wouldn't be available for use without the application of organic chemistry which brings about the production of such substances? As good as this sounds, it will be meaningless without proper knowledge of what organic chemistry is.
What is Organic Chemistry?
Organic Chemistry in a layman language is the chemistry of carbon and its compounds. Organic Chemistry is the branch of chemistry that deals with the structure, properties and reactions of organic compounds which contain carbon. The carbon containing compounds include carbon tetrachloride(CCl4), methane(CH4), pentane(C5H12), e.t.c.
We have three(3) large members that comprises the organic chemistry, which are;
- The Alkane
- The Alkene
- The Alkyne
For the sake of this post, I will explain only alkane and continue in my subsequent posts
ALKANE
Alkane has the general formula CnH2n+2 where n is the number of carbon atoms present. For instance, the first member of alkane group METHANE has 1 carbon, therefore, the molecular formula will be C1H(2X1)+2 which is CH4.
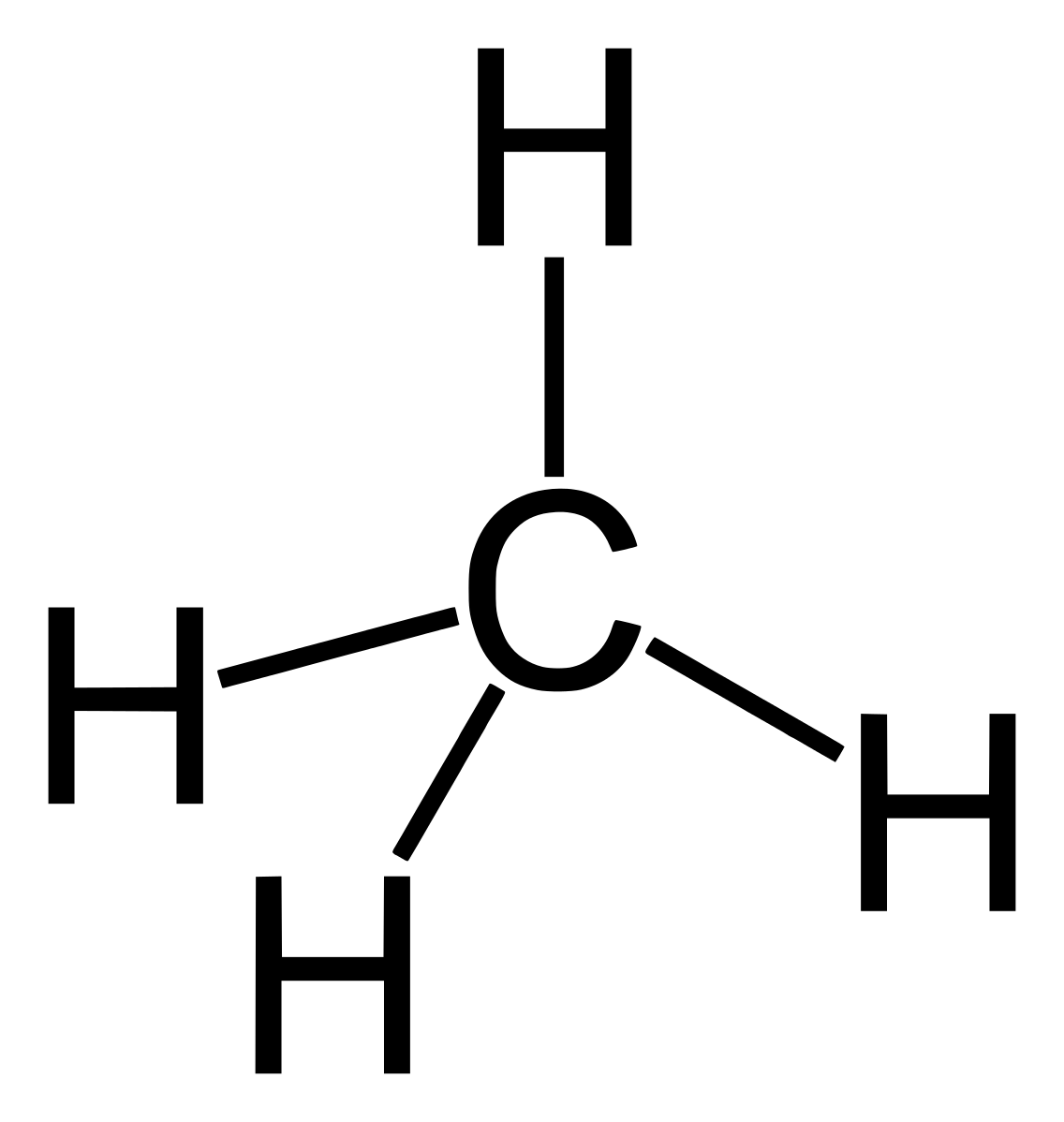
Alkane members are saturated hydrocarbons because they contain single carbon-to-carbon bond. This single bond is called sigma bond. The presence of not more than one bond between carbon atoms makes it alkane. Alkanes are SP3 hybridized.
Organic compounds structure can be in form of kekule structure or line segment. The kekule structure is the most popularly structure common for presenting organic compounds just like the structure of methane above. Line segment on the other hand is the use of lines to represent organic structure. It is usually jokingly called skeleton structure. Example is seen in butane below
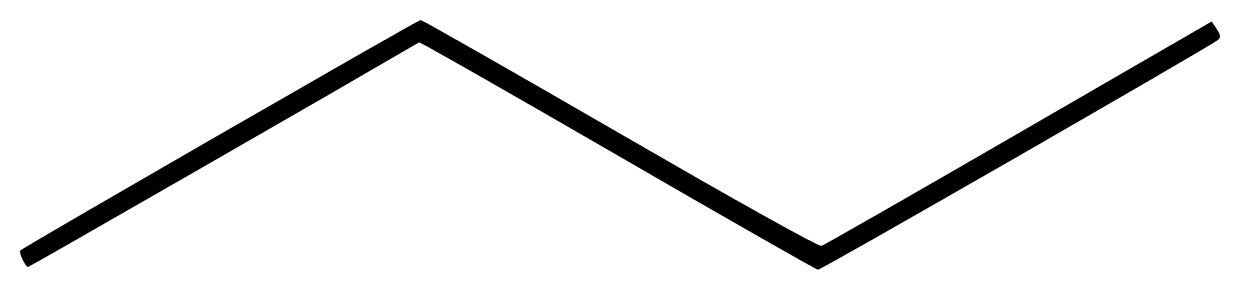
The edges of the lines represent carbon atom and four is present above.
RULES OF NUMENCLATURE(NAMING) IN ALKANE
For proper naming of organic compounds, there are some rules to babe followed.
1.) Locate the longest carbon chain.
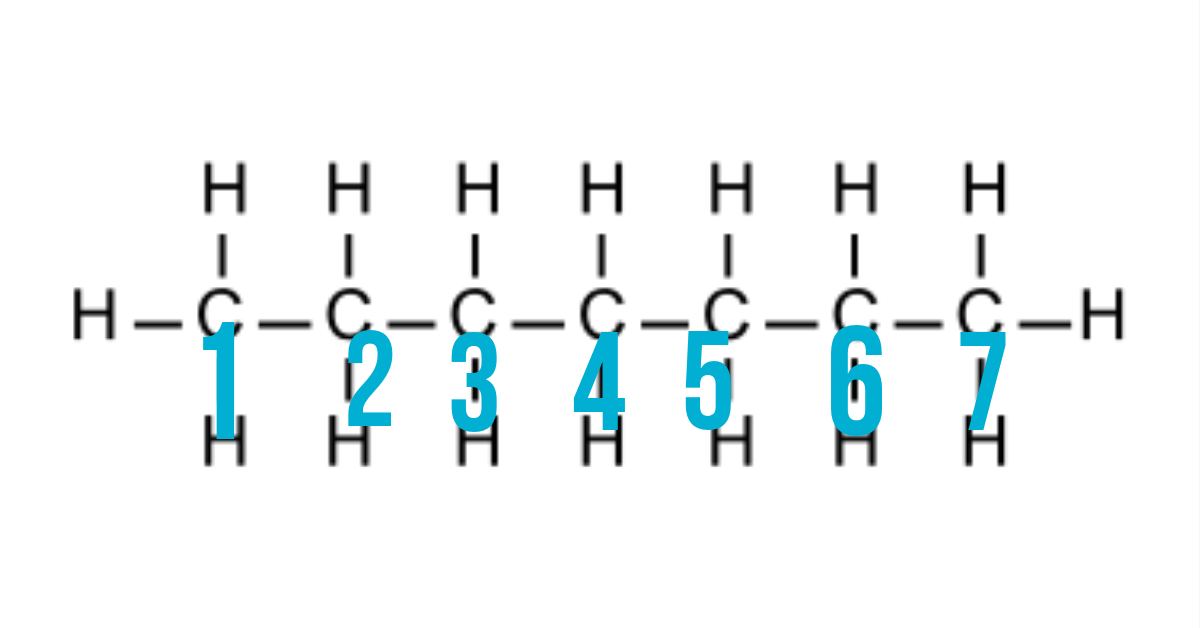
From the diagram of heptane about, our longest carbon chain is 7 carbon joined together as numbered there. Some structure may not be as straight as the one above but always note that, you should locate the longest carbon chain.
2.) Look for the attachment if any and note them.
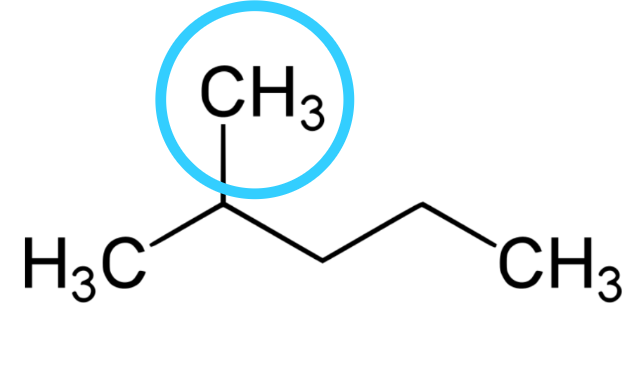
Any other thing attached to the carbon except hydrogen is regarded as attachment here. We can see CH3 is attached to carbon-2 which makes it an attachment. You can see that there is a lose of one hydrogen from the normal methane(CH4) we used to have, the group this attachment belongs to is alkyl group. So, the name of the attachment is methyl taking the last two letter of the family name -yl.
3.) The position of the attachment should be noted.
The attachment above is on the second carbon atom, that is why it is named 2-methylpropane.
4.) Number of attachment should be based on lowest possible number, therefore, the position of the carbon carrying the attachment will determine if it will be named from the front or back.
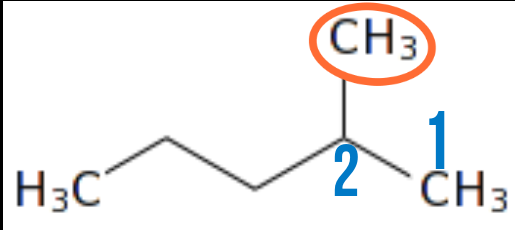
This structure is still the same with the last one expect that the location of the attachment is changed from the second carbon from the front to the fourth carbon from the front but, it still retains its name. In a case like this, we will count from the back because, that is where we will get the least number of the location of attament we are looking for.
5.) If the attachment is more than one, it is important to number it based on the carbon that gives the lowest possible number.
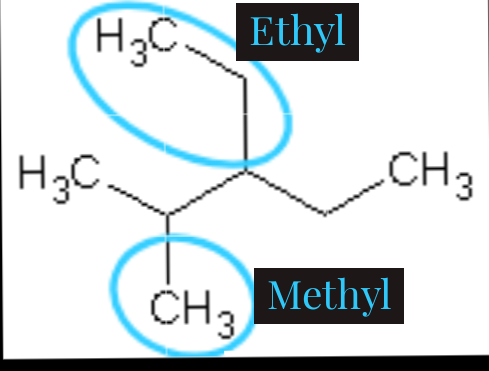
As we can see here, there are two attachments circled which is ethyl (C2H5) and methyl(CH3). If we count from the front, we will have methyl on carbon-2 and ethyl on carbon-3 while ethyl will be on carbon-3 and methyl on carbon-4 if we count from the back. As we are expected to count from where we will have the smallest number possible, we will count from the front.
6.) Arrange alphabetically.
When we have more than one attachment in an organic compound, we are expected to arrange it alphabetically when writing. Just like the example above, ethyl comes first before methyl if arranged alphabetically. So, the naming will be 3-ethyl-2-methylpentane. Because it has five carbons apart from the attachment, that's why it is named pentane.
SOME IMPORTANT THINGS TO NOTE WHEN WRITING NAME OF ORGANIC COMPOUNDS
- Every figure must be followed by an hyphen(-).
- If there is more than one figure at a time, there will be comma(,) between those numbers. For instance, 2,2-dimethylheptane.
- If a figure is at the middle of the name(i.e it us not the first figure), it will have hyphen(-) at the front and back. For example, 3-ethyl-2-methylpentane. Here, the 2 is not at the beginning of the naming which made it have hyphen at the front as well as back.
- After writing the attachment, the base name follows immediately without leaving any space.

USES OF ORGANIC COMPOUNDS
Organic compounds can be used for making
1.) drugs/medicines.
2.) wines and alcohol using ethanol.
3.) detergent.
4.) fuel for domestic purposes.
5.) soaps using hydrolysis of esthers using NaOH.
6.) making perfume and assence of soaps.
References
Introduction to Organic Chemistry
What are the main uses of Organic Compounds?

@tipu curate
Upvoted 👌 (Mana: 36/45)
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider supporting our funding proposal, approving our witness (@stem.witness) or delegating to the @stemsocial account (for some ROI).
Please consider using the STEMsocial app app and including @stemsocial as a beneficiary to get a stronger support.
My favourite topic is organic chemistry. Thank you for explaining
We are on this platform to help each other. Hope it has added to your knowledge?
Congratulations @sirpee6! You received a personal badge!
You can view your badges on your board And compare to others on the Ranking
Do not miss the last post from @hivebuzz: