Greetings to everyone! Haloalkanes, those intriguing compounds with halogen buddies hitching rides on carbon atoms, are like the undercover heroes of organic chemistry. These are also known as alkyl halides and are a class of organic compounds characterized by the presence of halogen atoms (fluorine, chlorine, bromine or iodine). They have halogen atom or atoms attached to a sp3 hybridized carbon atom of an alkyl group. They're found in everything from medicines to plastics, quietly playing crucial roles in countless everyday items. Today, we shall learn about their properties and see some of their methods of preparations.
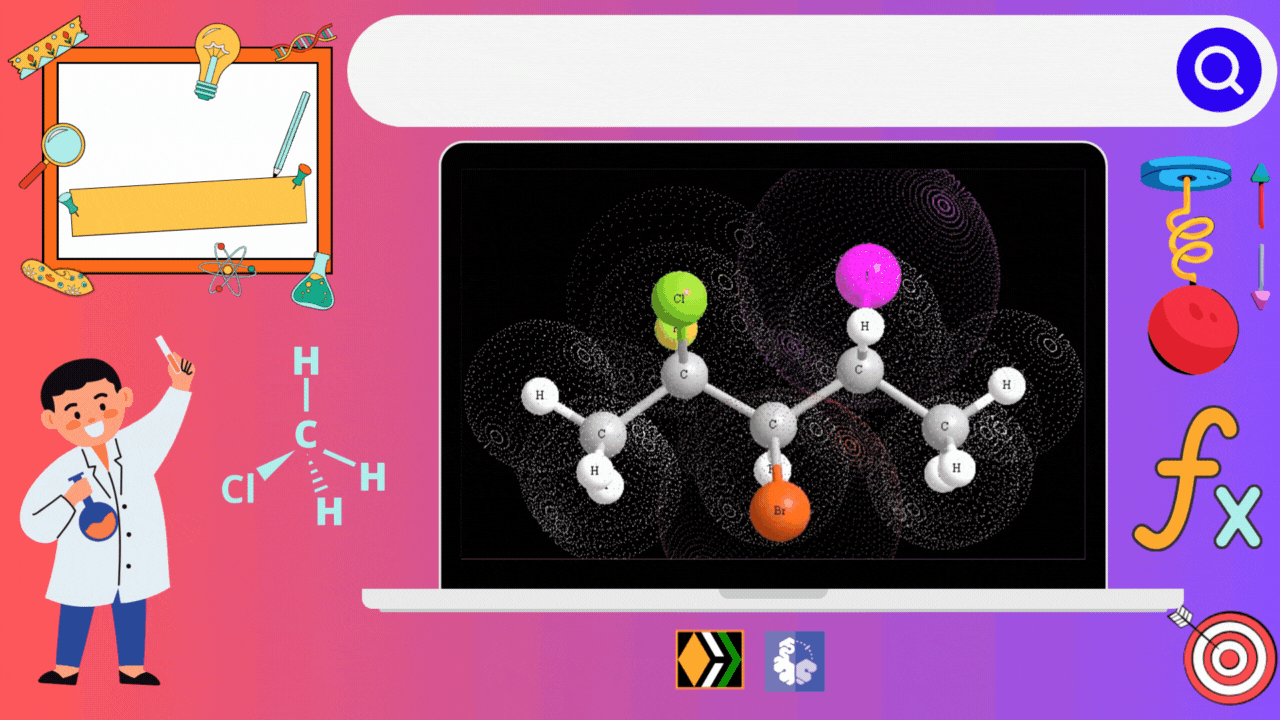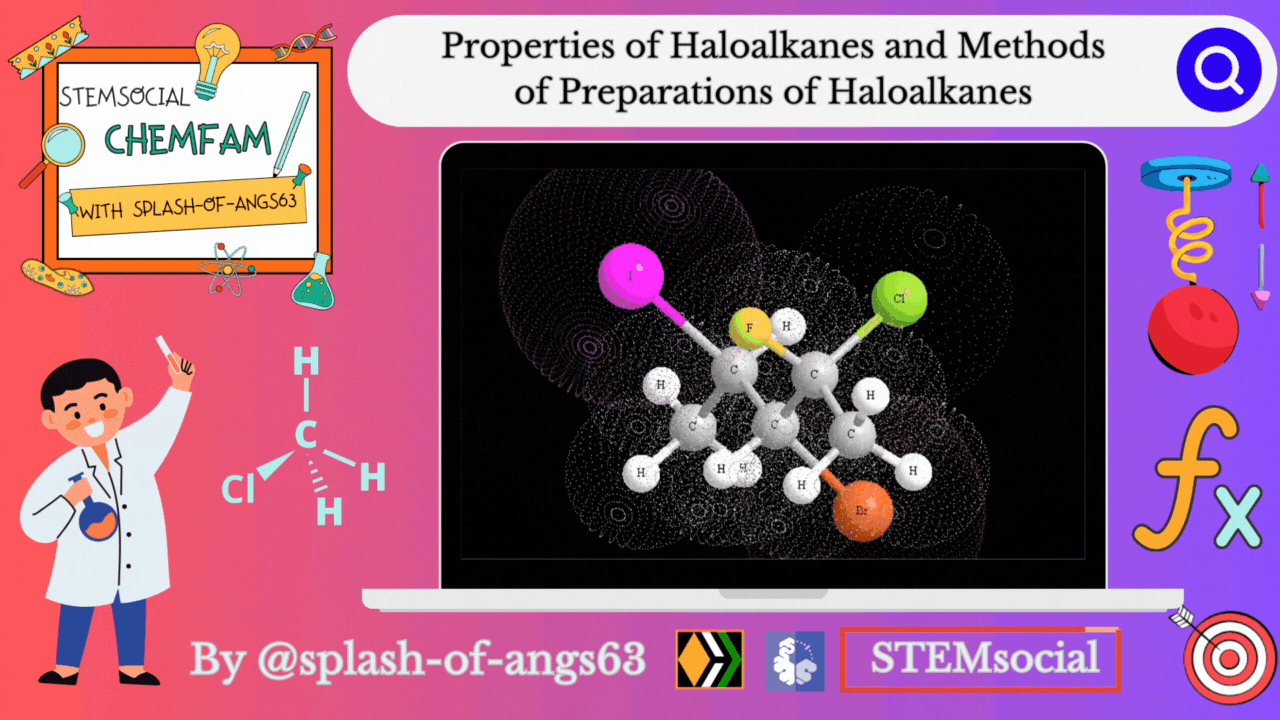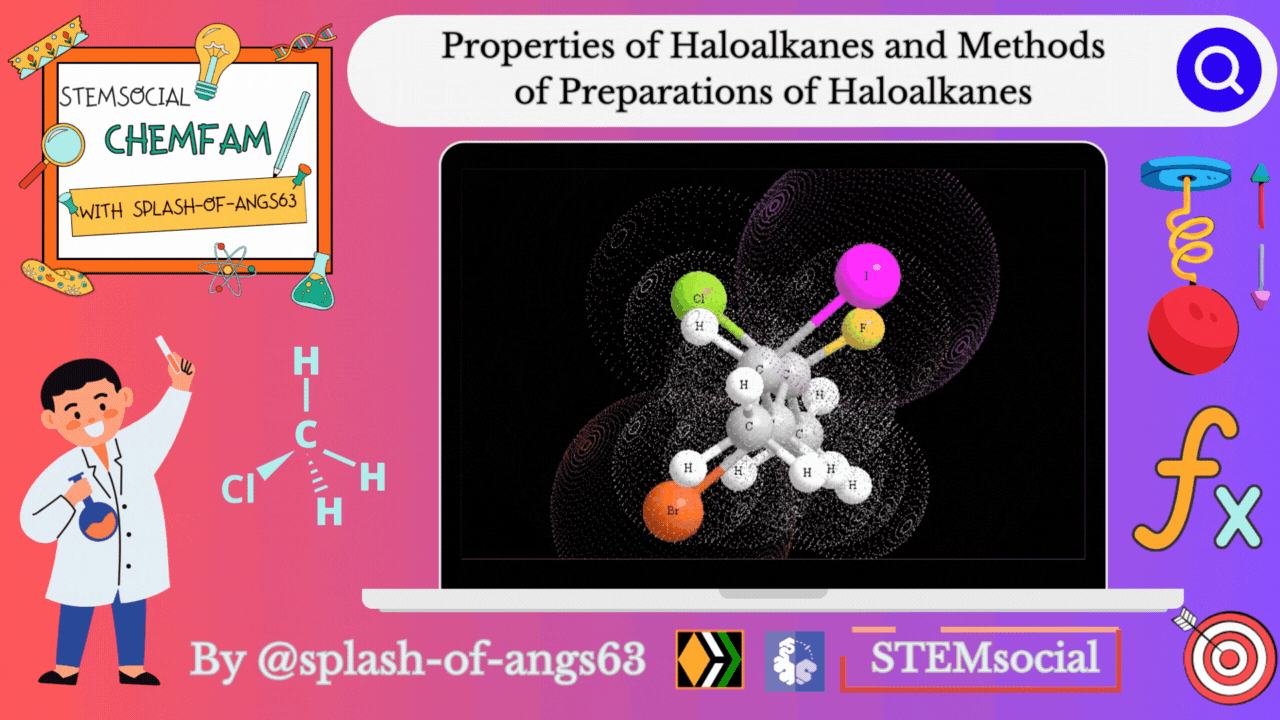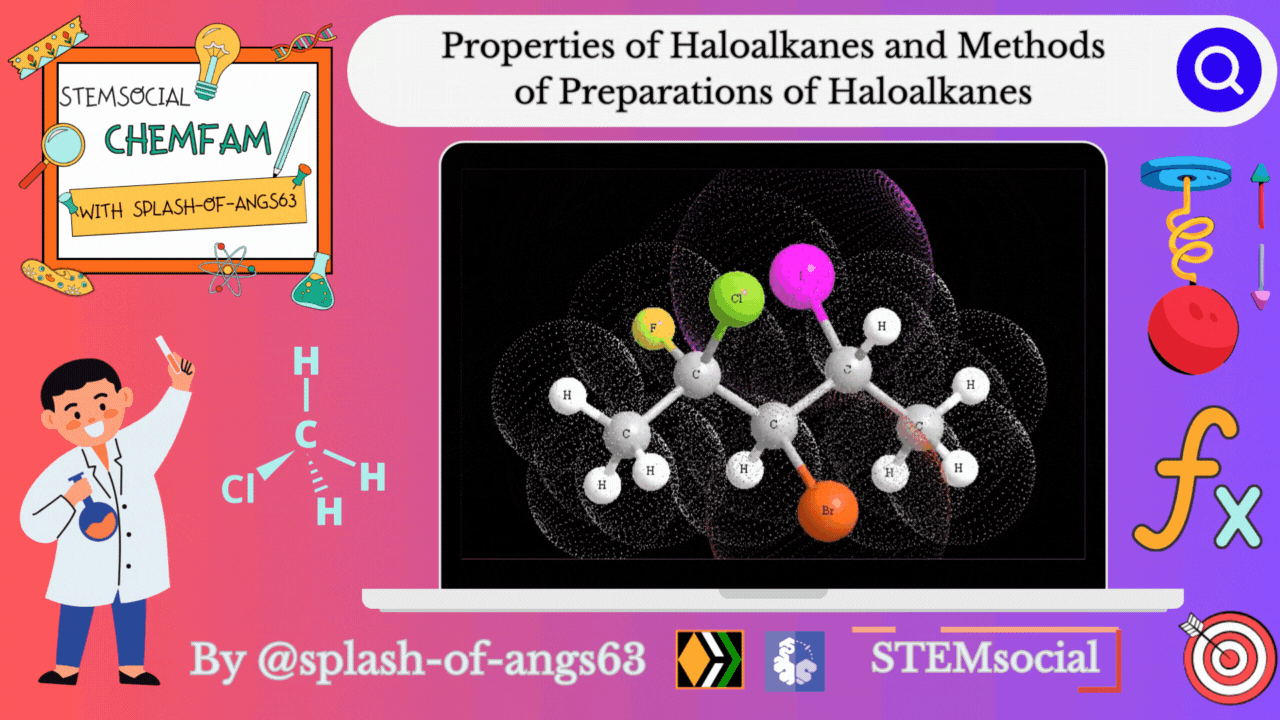
Properties of Haloalkanes
Physical properties
Alkyl halides have a higher boiling point than those of hydrocarbons with comparable molecular mass. This is due to the strong intermolecular attraction resulting from the polarity of the alkyl halide. For the same alkyl group, the boiling point (bp) of alkyl halides decreases in the order R-I > R-Br > R-Cl > R-F. This is because as the size and mass of halogen atoms decrease, the van der Waals forces decreases. In polyhalogen compounds, the boiling point depends on the molecular weight and varies in the following order: CCl4 > CHCl3 > CH2Cl2 > CH3Cl. The boiling points of isomeric haloalkanes decrease as the number of side chains increases.
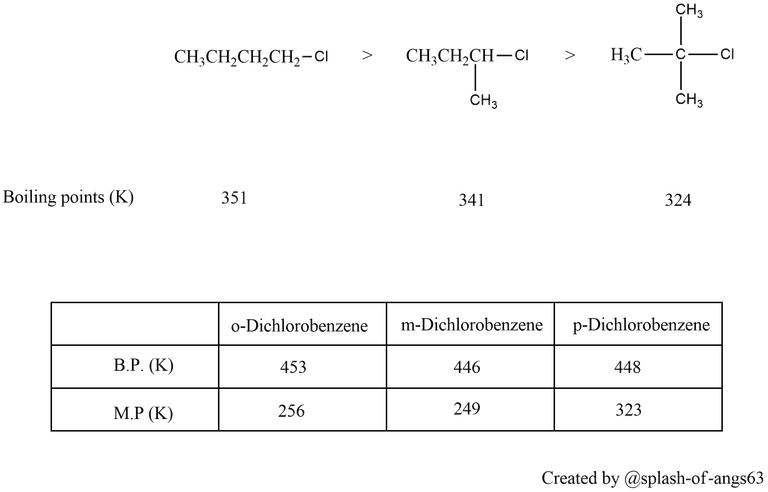
Isomeric dihalobenzenes have nearly the same boiling points. However, due to symmetrical structure of the para isomer, they get packed more compactly and hence have higher melting points than the ortho and the meta isomer.
Haloalkanes are not soluble in water as they are unable to form H-bonds.
Chemical Properties
Haloalkanes are no wallflowers when it comes to chemical reactions. They're game for swaps, kicks, and turns, participating in a dance of substitutions, eliminations, and cozying up to metals to form special compounds. Their behavior depends on who they're dancing with and where they are, making them the life of the organic chemistry party.
The C-X bond haloalkanes is polar with partial positive charge on carbon and partial negative charge on halogen (halogens are more electronegative than carbon). Any nucleophile stronger than the nucleophilicity of halide ion can attack at the electropositive carbon atom causing nucleophilic substitution reactions.
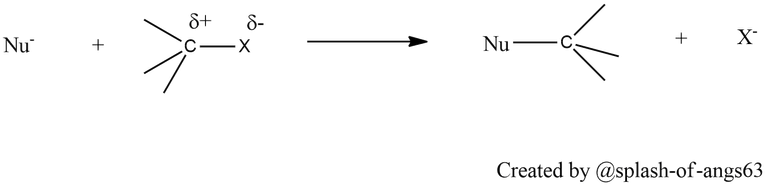
Being weak bases, halide ions are stronger bases and hence are good leaving groups. Thus they can undergo elimination reactions with a strong base.
Methods of Preparations
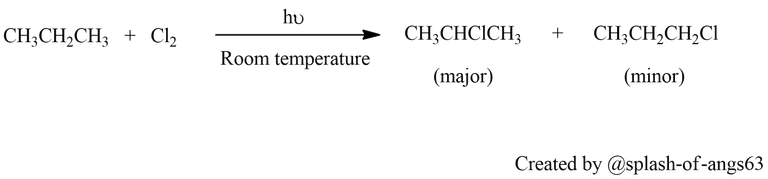
Halogenation of Alkanes: One of the primary methods for preparing haloalkanes is the halogenation of alkanes. This process involves the substitution of one or more hydrogen atoms in an alkane molecule with halogen atoms in the presence of a halogenating agent such as chlorine or bromine. The reaction is typically carried out under UV light or heat to initiate the substitution reaction.
Hydrohalogenation of Alkenes: Haloalkanes can also be synthesized via the addition of hydrogen halides (HCl, HBr, HI) to alkenes, a process known as hydrohalogenation. In this reaction, the pi bond of the alkene is broken, and the halogen and hydrogen atoms are added across the double bond to form a haloalkane. The reaction follows Markovnikov's rule, where the hydrogen atom adds to the carbon atom with the most hydrogen substituents.
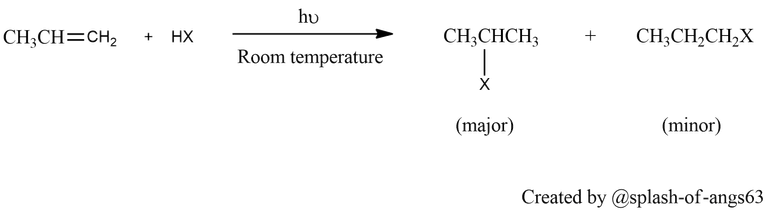
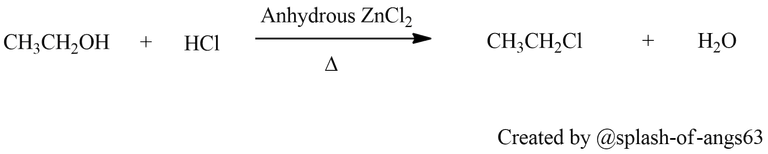
From Alcohols: Haloalkanes can also be synthesized from alcohols through substitution reactions. In this method, an alcohol reacts with a hydrogen halide (HCl, HBr, HI) in the presence of a Lewis acid catalyst such as zinc chloride (ZnCl2) to form a haloalkane and water. The reaction follows an SN1 or SN2 mechanism depending on the structure of the alcohol and reaction conditions.
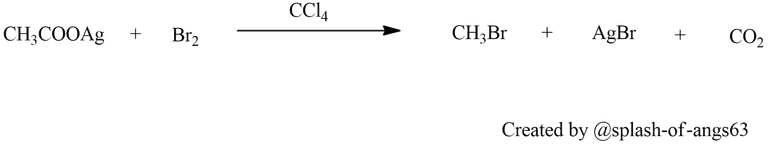
From Carboxylic Acids: Haloalkanes can be obtained from carboxylic acids through the reaction with thionyl chloride (SOCl2) or phosphorus pentachloride (PCl5). These reagents convert the carboxylic acid into an acyl chloride intermediate, which then undergoes nucleophilic substitution to form the corresponding haloalkane.
Software used:
The mathematical equations are prepared using mathcha.io editor and diagrams are drawn using ChemDraw software.

If you like my work and would like to support me, you can do so by joining my fanbase by clicking this link
Haloalkanes and Haloarenes | NCERT
Methods of preparation of Haloalkanes and Haloarenes | Geeksforgeeks
Understanding the Simple Harmonic Oscillator in Quantum Mechanics | ChemFam #86 |
Understanding Degeneracy in Quantum Chemistry: Exploring 1D, 2D and 3D Box Models | ChemFam #85 |
Introduction to Zero Point Energy and its Cases in Particle in a Box | ChemFam #84 |
Quantum Confinement : Particle in a 2D box and 3D box | ChemFam #83 |
Molecular Chirality: A Mirror Image Perspective | ChemFam #82|
Exploring Time-Independent Schrödinger's Wave Equation and Particle in a 1D Box | ChemFam #80 |
The Role of Gamma Function in Quantum Mechanics | ChemFam #79 |
Postulates of Quantum Mechanics and Normalization of Wavefunction |ChemFam #78|
Understanding Commutator Relations and Exploring Eigenfunctions in Quantum Mechanics |ChemFam #77|
How to find Expression of an Operator and Commutation Relations |ChemFam #76|
Basics to Quantum Chemistry: Operators, Functions and Properties of Operators |ChemFam #75|
A Comprehensive Study of Euler's Reciprocal Rule in Thermodynamics |ChemFam #73|
A Deep Dive into Nutrition Essentials: Your Path to a Healthier, Happier You |ChemFam #72|
Decoding Liver Function Tests through Chemistry |ChemFam #71|
Understanding the Dynamic Roles of Metalloenzymes and Metal-Activated Enzymes |ChemFam #70|
Cracking the Thermal Code: Differential Thermal Analysis in Modern Research |ChemFam #69|
Applications and Importance of IR Spectroscopy: Shedding Light on Molecular Structures |ChemFam #68|
PS The thumbnail image is being created by me using canva.com
Games I play on Hive
| Games I play on Hive | Game description |
|---|---|
| Terracore | Terracore is an Idle mining game based on Hive blockchain |
| Rise of the Pixels | ROTP is a Web3 browser game about game development on Hive |


!HBIT
!hiqvote
(1/1) tools | trade | connect@splash-of-angs63 mined HBIT. ⛏️
Made with LUV by crrdlx.
Discord Server.This post has been manually curated by @bhattg from Indiaunited community. Join us on our
Do you know that you can earn a passive income by delegating to @indiaunited. We share more than 100 % of the curation rewards with the delegators in the form of IUC tokens. HP delegators and IUC token holders also get upto 20% additional vote weight.
Here are some handy links for delegations: 100HP, 250HP, 500HP, 1000HP.
100% of the rewards from this comment goes to the curator for their manual curation efforts. Please encourage the curator @bhattg by upvoting this comment and support the community by voting the posts made by @indiaunited..
This post received an extra 1.06% vote for delegating HP / holding IUC tokens.
@splash-of-angs63, the HiQ Smart Bot has recognized your request (1/3) and will start the voting trail.
In addition, @splash-of-angs63 gets !PIZZA from @hiq.redaktion.
Discord. And don't forget to vote HiQs fucking Witness! 😻For further questions, check out https://hiq-hive.com or join our
$PIZZA slices delivered:
(2/5) @hiq.smartbot tipped @splash-of-angs63
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
Thanks for including @stemsocial as a beneficiary, which gives you stronger support.