Young's experience for electrons, in particular the formation of an interference pattern even when the electron beam is so thin that there is no doubt that electrons arrive one by one on the screen shows that the physics of electrons is incompatible with the concept trajectory.
Not exist in quantum mechanics, the concept of path
This is the content of the uncertainty principle, one of the foundations of quantum mechanics, discovered by Werner Heisenberg in 1927.
The way to get information about a quantum system (which we will call, for simplicity, electron) is to carry out interactions between him and classical objects, called appliances. Hypothetically these devices can be described by classical mechanics with the precision we want. When an electron interacts with a device, the latter state is modified. The nature and magnitude of this change depends on the electron state, and serve, therefore, to characterize it quantitatively. The interaction between the electron and the device is called the measure. A device need not be macroscopic. The motion of an electron in a Wilson chamber is observed through the nebula path he leaves; the thickness of this path is large compared to atomic dimensions. When the trajectory of an electron is measured on the low precision, it is an entirely classical object.
Quantum mechanics, at least in its current stage, occupies an unusual place among physical theories: it contains classical mechanics as a limiting case, and at the same time, needs this limiting case to establish their language.
The typical problem of quantum mechanics is to predict the outcome of a measurement from the results of a certain number of previous measurements. Moreover, we will see later, in comparison to classical mechanics, the quantum mechanical constrains the values of the measured physical quantities (e.g., energy). The methods of quantum mechanics allow the determination of these permissible values.
The measurement process in quantum mechanics has a very important property: the measure always affects the measured electron, and it is impossible, for reasons of principle, make the effect of the measure on arbitrarily small electron (as can be assumed in classical physics). The more accurate the measurement, the stronger the effect on the electron, and it is only in measures of poor accuracy that the effect of the measure on the electron can be considered small.
It is one of the fundamental postulates of quantum mechanics that the coordinates, the position of an electron can always be determined with arbitrary precision 2. Suppose that, at defined intervals 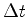 successive measurements of the coordinates of an electron are made. The results will not be, in general, on a smooth curve. Conversely, the lower the value of
successive measurements of the coordinates of an electron are made. The results will not be, in general, on a smooth curve. Conversely, the lower the value of 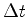 more discontinuous and disorderly will be the results, according to the fact that there is no path for the electron. A fairly smooth path is only obtained if the electron coordinates are measured with little precision as in the case of a cloud chamber.
more discontinuous and disorderly will be the results, according to the fact that there is no path for the electron. A fairly smooth path is only obtained if the electron coordinates are measured with little precision as in the case of a cloud chamber.
If, keeping charged the accuracy of position measurements, the intervals diminish 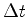 Among the measures, then adjacent measures will neighboring values to the coordinates. However, the results of a series of successive steps, albeit in a small region of space, will be distributed in this region, a totally irregular shape, and never on top of a smooth curve. In particular, when
Among the measures, then adjacent measures will neighboring values to the coordinates. However, the results of a series of successive steps, albeit in a small region of space, will be distributed in this region, a totally irregular shape, and never on top of a smooth curve. In particular, when 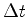 ends to zero, the results of measurements of adjacent any way tends to be on a straight line. Now the speed has the direction of the line that, in classical physics, is obtained in this limit. This fact shows that in quantum mechanics, there is the particle velocity in the classical sense, that is, the limit of
ends to zero, the results of measurements of adjacent any way tends to be on a straight line. Now the speed has the direction of the line that, in classical physics, is obtained in this limit. This fact shows that in quantum mechanics, there is the particle velocity in the classical sense, that is, the limit of 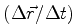 When
When 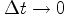 .
.
While, in classical mechanics, the particle has position and velocity well defined at each instant, in quantum mechanics the situation is quite different. If, as a result of a measure, to determine the coordinates of an electron, so its speed is completely undefined. If, however, determines the speed of an electron, then it may not have a defined position in space. Thus, in quantum mechanics, the position and velocity of an electron is low that can not be simultaneously set values.
@shirinkhan12 I see you sincerely are trying to convey certain fundamental concepts of QM. It's a bit hard to parse because it clearly wasn't written by someone for whom english is a native language.
I'll give you an A for effort here.
But you should be advised that I have a bot that scans the blockchain looking for content based on a "conceptual similarity index".
This looks an awful lot like you took the english wikipedia article on the double slit experiment, tried to translate it to your native language and then tried to explain certain parts verbatim. Possibly just translating in and out directly.
The conceptual flow is certainly a 1:1 for the original wikipedia article...
https://en.wikipedia.org/wiki/Double-slit_experiment
I'll give you the benefit if the doubt if you can explain the relationship between the math terms you inserted, which involve delta r over delta t.
If you're sincerely interested in the topic of quantum mechanics and not just trying to slip some content past @cheetah and @anyx please feel free to join us in discussing some exciting new developments in this field. https://steemit.com/science/@williambanks/can-history-be-rewritten
Have a great day!
Good thoughts
Congratulations @shirinkhan12! You have received a personal award!
Click on the badge to view your own Board of Honor on SteemitBoard.
For more information about this award, click here
Congratulations @shirinkhan12! You received a personal award!
You can view your badges on your Steem Board and compare to others on the Steem Ranking
Vote for @Steemitboard as a witness to get one more award and increased upvotes!