The photoelectric is perhaps one of the most influential discoveries in human history. It paved the way for solar power which is readily used today. Many people all around the world use and rely on this effect to power their homes and appliances, but most people don't have a grasp of what it actually is.
(a good source to explore photoelectric effects is here
The effect.
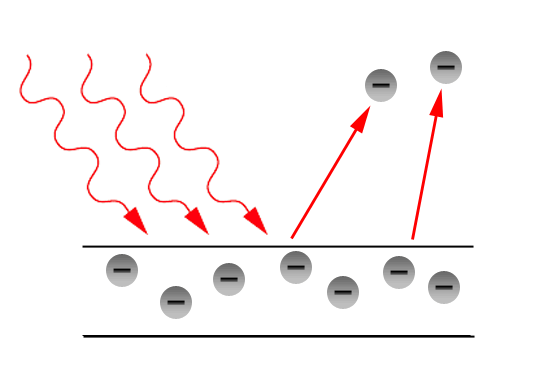
The photo electric effect is the name for when light shines on a material and it releases electrons and free carriers. This is because when the light hits the surface of and it dislodges the electrons from the photoemissive material.
The photoelectric effect was first truly tested by Philipp Lenard. What he did was, he cleaned the surface of a piece of metal and put it into a vacuum. The metal plate was then set in evacuated glass chamber with another metal plate not touching it but on the other side. The photoemissive plate was then positioned to have a light shown on it. There would then be a wire connected to each of the plate and connected them to a variable power supply, micro-ammeter and a voltmeter. He then shined light onto the surface of the photoemissive plate and collected the results.

The idea was to see if the light would knock electrons from the plate. The free electrons would float about in the chamber, and the plate that they came from would become slightly positive in charge. The other plate on the other side would also then be positive and attract those electrons to it where they would hit it and then go back to the plate that they originated from.
The electrons that flow from one plate to the other and it creates a current. The current is then measured using the macro-ammeter. This can then be used to find how many electrons are actually leaving the surface of the photoemissive plate.
In the experiment, the photoemissive plate is hooked to a power supply with the negative wire is attached to it. This creates a potential difference that will push the electrons back to the photoemissive plate. Because of this the power supply can be set create different levels of potential difference. The electrons that are shot are effected by this potentoial difference and the ones that have less energy cannot make it to the other plate, this in turn reduces the current detected by the micro-ammeter. This process allows you to measure the stopping potential of the electron and the kinetic energy of the electron.

Another interesting aspect of the photoelectric effect is with the intensity of the light and the wavelength. The intensity of the light as in how bright it is, does not effect the current. Only the wavelength of the light does. For instance, red light will almost never knock electrons off the plate. but violet light will. This is because the wavelength of violet light is much more energetic than yellow light. This also means that the ejected electrons that were exposed to bright light have the same energy as the ones ejected from the dim light with the same frequency. But this does mean that with the conservation of energy, that more electrons are ejected from a bright light than a dim one.

Sources:
upload.wikimedia.org/wikipedia/commons/7/77/Photoelectric_effect.png
pixabay.com/static/uploads/photo/2013/07/12/14/08/light-147810_960_720.png
pixabay.com/static/uploads/photo/2016/02/25/17/31/atom-1222511_960_720.jpg
upload.wikimedia.org/wikipedia/commons/9/92/Gallium_crystals.jpg
Hi,
Talking about the photoelectric effect without talking about Einstein who explained it and got a Nobel prize for it? Hmm...
And what about the minimal work that must be provided to extract a photoelectron?
What about the fact that the electron kinetic energy is proportional to the frequency of the photon.
Maybe some quantum mechanics?
Sorry for complaining, but I believe your post can be improved by a lot! Please give it a try!
thanks for the info... so thats how the solar panels on my roof produce energy.... i wish the sun shine violet all day long...