How Phase Change Materials (PCM) Works
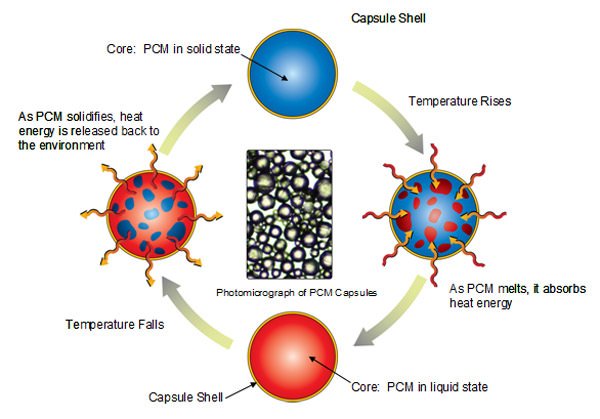
Phase change materials (PCM) have received considerable attention over the last decade for its use in latent heat thermal storage (LHTS) systems. They use chemical bonds to store and release heat. Phase Change Material (PCM) is a substance with a high heat of fusion which melts and solidifies at certain temperatures and is capable of storing or releasing large amounts of energy.
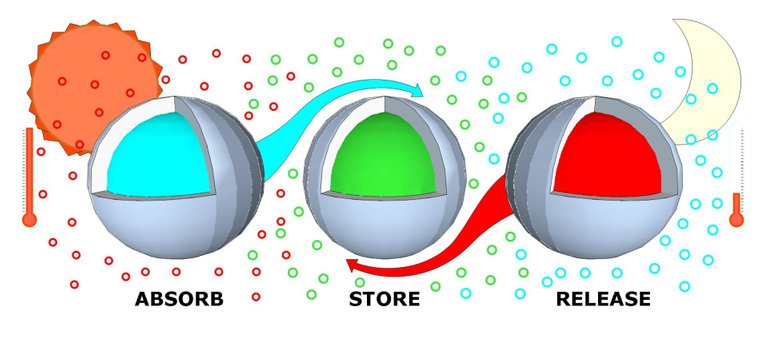
Phase change materials (PCM) are substances that have the ability to change phases at certain temperature range and due to its ability, current studies uses PCMs for temperature stabilization and for storing heat with large energy densities.
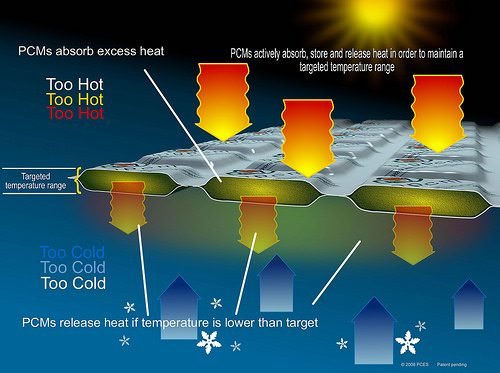
Phase change materials (PCMs) are organic or inorganic compounds, which melts and solidifies at a definite level of temperature suitable for specific applications. They have the ability to absorb and release large quantity of heat during phase change. However, PCM has some deficiency that result to some problem in their application, including low thermal conductivity, high volume change, and liquid ramps during the transition phase. Therefore, to effectively use these materials, they need to be contained in an inert and highly durable capsule. Current studies shows that there are several solutions found out regarding its containment including the engagement of supporting materials, such as polymers or porous materials, for stabilizing the compound present in the phase change materials to which is known as microencapsulation. Microencapsulation is shown to provide an effective encapsulation of PCM. Microencapsulation, through an increasing heat transfer area, reduces PCM reactivity to the outside environment and prevents the PCM to leakage when it is in liquid state.

According to Latibari and its member, the traditional microencapsulated PCMs were fabricated using polymeric wall materials, such as melamine formaldehyde resin, urea formaldehyde resin, polyurethane, and polystyrene. However, there are several defects, such as flammability, poor thermal and chemical stabilities, and low thermal conductivity, for these microencapsulated PCMs because of the polymeric shells. On the other hand, the thermal conductivity of inorganic materials is found out to be higher than organic materials. Its fire resistance and also the chemical and thermal stability are superior to those of organic materials. Hence, some attempts have recently been taken to enhance the thermal conductivity and mechanical strength of the microcapsules by encapsulating PCMs with inorganic wall material.
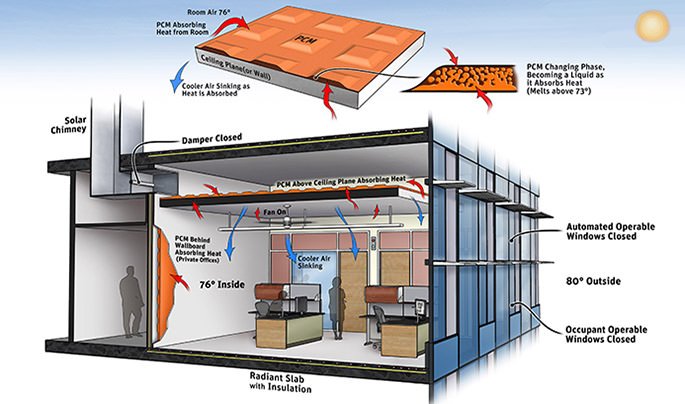
This incorporation of PCM in the energy storage devices of the industry is a great way to enhance the energy storage capacity.

