
While new technologies are often welcomed by the public with a mix of excitement and legitimate suspicion, few have raised as many questions and speculations as the CRISPR technology since its first use in 2012 at the University of California in Berkeley.
Most people have heard of genetic engineering, and while some have strong opinions on the subject, recent studies have shown that few have a clear idea of how tools like CRISPR work and what they are used for. A recent report from the Genetic Alliance UK and the Progress Educational Trust, two non-profit organizations, have shown that among 86 participants in an online workshop on genome editing, all of them had heard the term “genome” before but roughly half of them thought that it referred to a small unit of genetic information and most of them were unable to define it precisely. This lack of understanding of the specific vocabulary used by scientists combined with sensationalist headlines and over-simplified affirmations often found in mainstream media contribute to the general misunderstanding on the topic. Independently of your opinion on genetically modified organisms, the technology is already part of your life: insulin pens, drought-resistant crops, control of mosquito populations, genetic selection in livestock, next-generation cancer treatments, biofuel production, biohacking... These are only few examples of the current democratization of biotechnologies. It is thus essential to have a healthy debate on these questions in our societies, and this is only be possible through education and an effort of transparency from the scientific community.
The goal of this article series is to expose some common misconceptions on biotechnologies in general and to provide simple keys of understanding on tools that were long restricted to science fiction novels and are now in the hands of scientists around the world. But first, we need to be on the same page on what we are talking about, so let's start with some basics about the now famous CRISPR system.
What is CRISPR?
CRISPR (short for Clustered Regularly Interspaced Short Palindromic Repeats) is already a relatively bad name for a technology predicted to change the lives of millions of people, but its full name is actually CRISPR/Cas9. The history of the CRISPR discovery is fascinating on many levels, from its origin in bacteria where it acts like an archaic adaptive immune system against viral infections, to the intricate patent war raging between UC Berkeley, the Broad Institute and other academic and industrial actors claiming the paternity of the technology. These aspects will be treated in future articles; we will here only focus on the CRISPR/Cas9 system itself and why it represents such an important advance in Biology.
CRISPR/Cas9 is a molecular system found in many microorganisms. It is composed of a protein called Cas9 associated with one or two RNA chains. RNAs (for Ribonucleic Acids) are abundant and ubiquitous molecules found in living cells, they share similar characteristics with DNA but display different physical and biological properties. Notably, while DNA molecules are generally composed of two strands forming the famous double-helix structure, RNA molecules are often single-stranded.
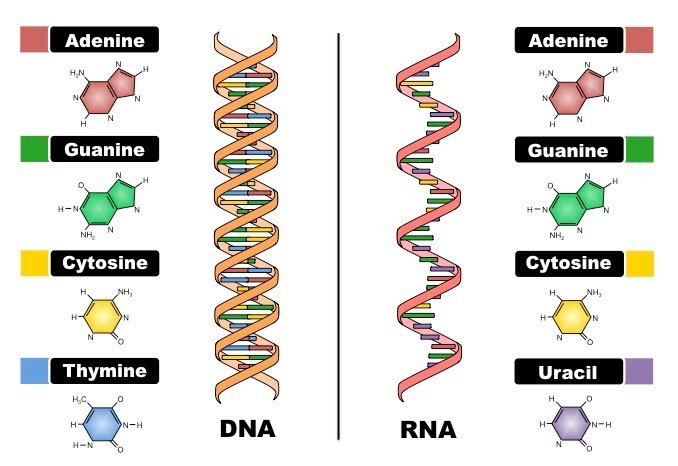
RNAs are, like DNA molecules, composed of molecular “letters” called nucleotides (A, T, G, and C in DNA and A, U, G and C in RNA) which, taken together, form the genetic information (or genome) stocked in the DNA of every cell of a considered organism. Interestingly, under certain conditions, the similarity of RNA and DNA molecules allow them to interact with each other thanks to the complementarity of their nucleotide sequences.
In the context of CRISPR/Cas9, the RNA component of the system is short and you can think of it as a photographic negative of a small section of genetic information contained in DNA. This means that this RNA, often referred to as guide RNA, can specifically interact with a defined region of a very large DNA molecule (such as a human chromosome or a viral genome) by recognizing its sequence. When associated with the Cas9 protein, the guide RNA can thus specifically target the protein to a specific DNA site. The Cas9 protein can then induces the chemical cleavage of the DNA double helix at this site, which is why the CRISPR system is often compared to “programmable molecular scissors”. Another analogy often used is that if the genome was a giant text file, the guide RNA would be the “find” function and Cas9 the “cut” function in Microsoft Word.
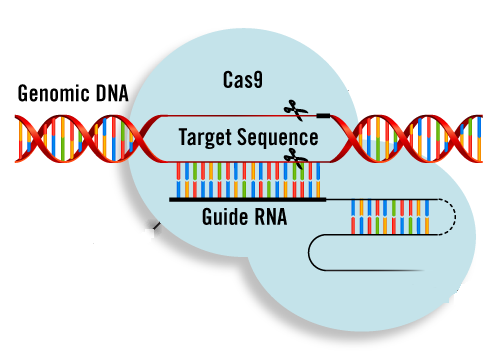
Presented like that, this might not sound that exciting. After all, researchers were already able to specifically cut DNA in vitro since the 70s with restriction enzymes (also isolated from bacteria) and later with higher accuracy in living cells with the artificial Zinc Finger Nucleases and TALEN proteins (naturally found in plants). These systems also involve a protein able to cut DNA but the recognition of the target site required an assembly of different protein “blocks”, each able to recognize a very small DNA sequence. Assembling multiple of these blocks together thus allows the recognition of a larger region. What makes CRISPR different from these systems is that it does not require the inefficient and cumbersome assembly of chimeric proteins. The Cas9 protein is indeed the same independently of the desired target, only the guide RNA differs and RNA molecules are infinitely easier and cheaper to produce synthetically or to produce directly inside a cell than proteins.
While what is achieved today thanks to CRISPR/Cas9 could thus theoretically be achieved with earlier technologies, CRISPR made the whole process easier and a lot more affordable, quickly allowing the democratization of genome engineering in countless labs around the world. If we consider the Microsoft Word analogy, one might argue that when you want to deal with a text file, the “find” and “cut” function are not enough. However, as we will see in the next article of this series, cutting DNA in the context of a living cell is enough for a lot of very interesting application.
Sources and recommended readings:
- The report of Genetic Alliance UK and the Progress Educational Trust: http://www.geneticalliance.org.uk/our-work/medical-research/communicating-about-genome-editing/
- A well-illustrated video on how CRISPR works and its implications for the future: https://www.youtube.com/watch?v=jAhjPd4uNFY
- A TED conference by Jennifer Doudna, one of the “inventors” of the CRISPR technology: https://www.youtube.com/watch?v=TdBAHexVYzc
awesome post! im a biologist teacher at one of school in Aceh, this article so helpfully me to teach about CRISPR for Biology Olympiad
Wow that's such a nice compliment, thanks a lot :) I'm glad this helped you to teach others, keep up the good work !
Great post, love it! I hope they overcome the ethical side of human uses of this before too long and we can really start seeins come changes!
Thank you for your comment. The ethical part is the main question i want to discuss in my articles, i just wanted to start with a quick introduction so i can use it as a reference for my future work.
Glad to hear it! Awesome :)
Great post! Resteemed. I'm looking forward to reading more ;).
Recently I have come across two really interesting articles regarding the CRISPR-Cas9 system. The first is connected with the potential immune response of the human body to Cas9 proteins. The currently used homologs are derived from Staphylococcus aureus and Streptococcus pyogenes, so it is possible that the pre-existing adaptive immune responses can occur due to the contact of human population with those bacteria.
Identification of Pre-Existing Adaptive Immunity to Cas9 Proteins in Humans
The second describes the amazing possibility to modify the epigenome by bounding the Cas9 with an additional catalytic domain.
The epigenome: the next substrate for engineering
Those are two great articles, thank you for sharing. There are countless Cas9 protein fusions that have been published in the past two years but I agree that the chromatin-remodeling or nucleosome modifiers are of particular significance since epigenetics studies still lack this kind of tools.
If you are interested in other applications, one that i am particularly excited about is the RNA editing by Cas13 mutants:
Thank you for your comment @pavlvus ! http://science.sciencemag.org/content/early/2017/10/24/science.aaq0180
Good to see introductions to CRISPR on here, and not the fear-mongering kind!
CRISPR/Cas9 is but one of the many CRISPR effectors though, with Cpf1 making much progress to becoming a more precise genome engineering tool. Though, this precision does come with a trade-off of overall enzymatic activity, and less ability to displace nucleosomes the way Cas9 does.
The part I find most remarkable about this is that the "scanning" of Cas9 (or Cpf1) for the proper target that matches the gRNA, target recognition, and hydrolysis of target DNA, is all ATP and NADH/NADPH independent. That such robust activity can occur without additional energy input is quite something. Once assembled, this system works remarkably, despite the chance of off-targets; but then again there are the new hyper-accurate Cas9 variants, or Cpf1 if specificity is an issue.
Looking forward to the next in the series, and if you have any questions, feel free to hit me up; I work with these tools and would relish the opportunity to blab on about my work!
Cheers!
Thank you for your comment! I also work in the CRISPR field so i'm definitely more excited than concerned about the technology :) I'm glad that you like the it, i'll be happy to have your feedback on future publications since i don't have a lot of experience in the writing of this kind of articles.
You raised some pretty interesting points here. I am not a Cpf1 expert but i think that a very interesting (and relatively unexplored) feature of it is that it produces a staggered cut, which could lead to different repair outcomes compared to Cas9. I think that in the long term we will see these different enzymes fit into a molecular toolbox that could be adapted to specific applications (longer or shorter PAM, cutting activity...). If you are interested in accuracy, here is a promising report about the Cas9 from Nesseiria Meningititis (still not peer-reviewed thought):
https://www.biorxiv.org/content/early/2017/08/04/172650
The energy question is also very interesting, I think the fact that DNA could be cleaved simply using the free energy stocked in the molecule must have been a critical reason of why it has become the support of heredity at the early stages of evolution, but this is just speculations at that point :)
@originalworks
Great article! Upvoted/followed/resteemed, and I'm anxiously waiting for the other posts of the series as well as an introduction post ;-)
Hi there, thank you very much for the support. Let me know if something is unclear, i'm still learning :)
good post, thank information
@steemcleaners
Im working in a biotech company and I also use CRISPR to modify genes. we hope that the ethical discussion will soon get a conclusion because when they dont we have problems getting our substances to the market in Germany.. I will follow your ethical subjects!
Hi @julyjules90, i also work in a biotech company. We both know how useful this technology is, that's why we need to make it transparent for everyone so the products we develop can be accepted by the public :)