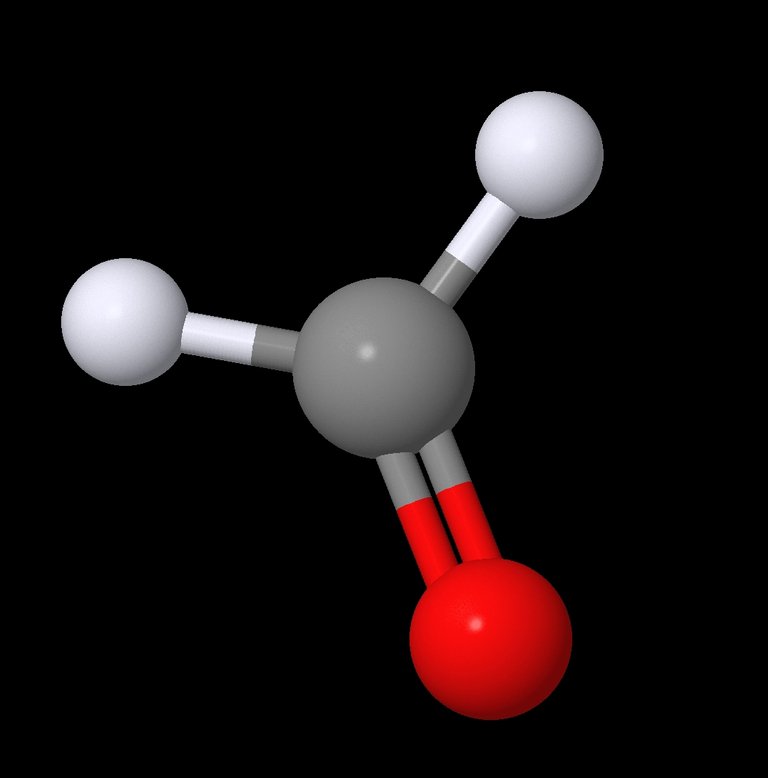
Ye old ball and stick model of Formaldehyde
PubChem Chemical ID: 712
Empirical Formula: H2CO or CH2O
Molecular Weight: 30.03 g/mol
Synonyms:
Formalin
Formol
Methanal
Oxomethane
Formaldehyde is a ubiquitous and water soluble, poisonous, carcinogenic and highly reactive gas with a profusion of industrial uses. Formaldehyde can be found in the production of resins and textiles, used as a disinfectant, in particle and fiberboard and a whole mess of other products . However, more commonly it as known as being one of several components in embalming fluid (1,2).
Unknown to many though, formaldehyde is also produced by most organisms, including humans as part of normal metabolism. It is in fact part of an essential process in Amino Acid metabolism and several others processes in our bodies. Breakdown of pectin in fruits and vegetables has been shown to produce methanol, which is further metabolized into formaldehyde. Turnover of formaldehyde in the human body has been estimated to be 878-1310 mg/kg a day (3). That’s about 70 to 105 grams for the average American daily! Aspartame, AKA Nutrasweet, is broken down into two endogenous amino acids, Aspartic Acid and Phenylalanine, and Methanol as well (4). Might wanna lay of those diet drinks eh? Well, maybe not, at least don’t kick yourself in the head for drinking one 12 oz can of diet coke, which contains about 186 mg of Aspartame, of which about 10% will convert to Formaldehyde (5). For perspective, I give you a list of food types and their formaldehyde yields.
Meat and poultry 5.7-20
Fish 6.4-293
Milk and milk products 0.01-0.80
Sugar and sweeteners 0.75
Fruit and vegetables 6-35
Alcohol beverages 0.27-3.0
*Formaldehyde content in milligrams per Kg! That’s per ~2.2 pounds!
After looking at this list, it becomes quite obvious that no matter what you eat, the amount of H2CO will be insignificant to what your body churns out daily. Lucky for us, our bodies and every other living thing on the planet are good at eliminating this carcinogen!
For this weeks chemistry lesson I will dabble in covalent bonds. Covalent bonds are basically when atoms share pairs of electrons. Formaldehyde in this case has 4 covalent bonds, 2 Carbon to Hydrogen single bonds and a double bond between Carbon and the Oxygen. Single bonds share 1 pair of electrons, doubles share 2 pairs, triple bonds share 3 pairs etc. An important thing to take away from this is that single bonds allow free rotation. Where double bonds only allow some slight twisting, and amount of twist depends on many factors. Double and triple bonds can make molecules and groups within them rigid.
(1) https://pubchem.ncbi.nlm.nih.gov/compound/formaldehyde
(3) https://efsa.onlinelibrary.wiley.com/doi/pdf/10.2903/j.efsa.2014.3550
(4) http://care.diabetesjournals.org/content/diacare/12/1/67.full.pdf
Congratulations @chemicalweek! You have completed the following achievement on the Steem blockchain and have been rewarded with new badge(s) :
You can view your badges on your Steem Board and compare to others on the Steem Ranking
If you no longer want to receive notifications, reply to this comment with the word
STOPVote for @Steemitboard as a witness to get one more award and increased upvotes!
This post has been voted on by the SteemSTEM curation team and voting trail.
If you appreciate the work we are doing, then consider supporting our witness stem.witness!
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
Please consider setting @steemstem as a beneficiary to your post to get a stronger support.
Please consider using the steemstem.io app to get a stronger support.