In this post I would like to give a layman's explanation of my primary scientific discipline, organic chemistry. This will be an introduction to a series of organic chemistry posts based around the concept of nanoscale architecture.
Organic Chemistry is Nanoscale Architecture
In architecture it is important to first have a frame to give a structure rigidity and strength. The frames are made up of beams and joints. Many towers, such as the Eiffel tower, show off these elements for everyone to see. When the Eiffel tower was built, very large beams were necessary to construct such a massive structure. However, a model Eiffel tower that fits on your coffee table would require beams the size of matchsticks. One could scale down further to make smaller and smaller Eiffel towers, but what is the limit? What are the smallest beams in existence?To find out we will have to zoom down to the very fabric off our universe, atoms. There is a special atom of which all life on earth requires due to it's flexibility as a structural atom. This atom is carbon. Carbon forms unusually strong bonds with other carbon atoms and as a result is excellent for building nanoscale structures. In its tetrahedral crystaline form, diamond, carbon is one the hardest materials on earth. In its planar form, graphene, it is highly conductive. Most importantly, every biomolecule in existence relies heavily on carbon for providing structure. What is so special about carbon and what are the tiny beams that hold carbon atoms together?
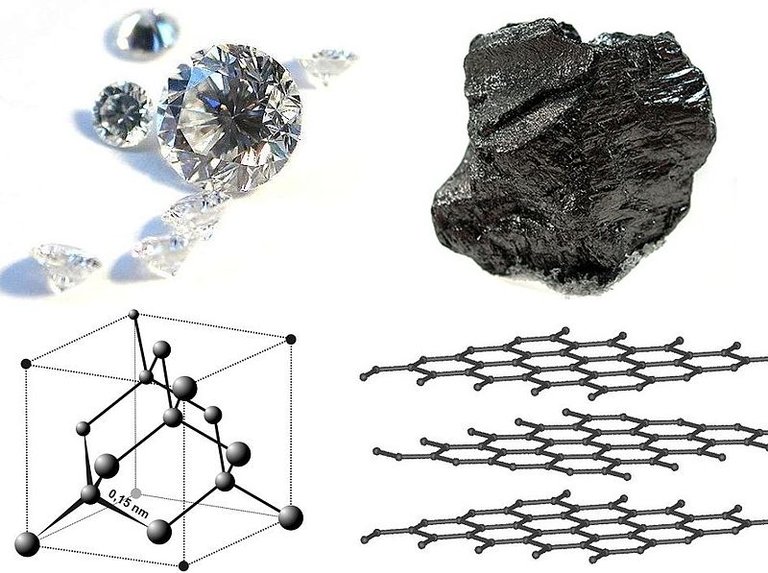
Each carbon atom can form four covalent bonds with other atoms. The simplest molecule of carbon is methane, where carbon is bonded to four hydrogen atoms. In these bonds, electrons are in both the carbon atom and the hydrogen atoms' orbitals which puts them in a lower energy state than if the atoms were separate. Despite being in a low energy state, methane is in a metastable state. This means they it is stable, but not in the theoretically most stable state. This is why organic molecules can be burned and transformed into water and carbon dioxide. As a result, each molecule is like a plateau on an energy landscape where the peaks of the mountains are reagents and water and carbon dioxide exists in the valleys.
This raises a very exciting question! What can we build? How can we combine atoms to create new structures? The possibilities are enticing. Throughout the years many exotic organic molecules have been synthesized. In this series I will explore some of the most intriguing molecules that have been made so far.


Hey mate, I must be below layman in my comprehension level. It all pretty much sounds like jibberish to me, interesting, but jibberish. :) I'm familiar with nanobots from the GI Joe "The Rise of Cobra" movie but that's about it. Here they are destroying Le Tour Eiffel.
I'll give you a follow and a RS on this post though and see where your posts lead over time. I like that you write about things that are important to you. That's the way to go I reckon. Keep up the good work.
Thanks, I'll focus more on making it understandable. I'm a graduate student and that is one thing that I need to work on.
*Crosses fingers for graphene *
So much excitement has been put behind graphene. It behaves really well as a conductor, but a big problem is making contacts to go from wires to the graphene and back again with little resistance.
:)
Hey there, this is a really interesting subject, and so it would be really worth your while to add some references. I understand it's your work, but for the interest of further reading and understanding (as the other comment here says, they see it as interesting jibberish!). That way you're more likely to get better recognition and curation rewards =D
Good luck!
Thanks for the feedback. I'll try to find some good further reading links in the future.
Think about your tags too mate. You have five, so make sure they match your topic and also target your intended audience.