By Jon CohenJul. 12, 2017 , 9:00 AM

ST. LOUIS, MISSOURI—A few months before completing medical school in 2003, Lukas Wartman was diagnosed with acute lymphoblastic leukemia (ALL), a blood cancer that's particularly lethal when it strikes adults. So began a battle to stay alive that has involved more than 70 drugs, two rounds of cell transplants, and a staggering series of twists and turns.
Wartman immediately received aggressive chemotherapy, which drove the cancer into remission and enabled him to graduate from Washington University School of Medicine (WUSM) in St. Louis in Missouri. He then embarked on a career of clinical care and lab research focusing on leukemia, the disease that had almost killed him. But 5 years later, ALL returned.
Another round of chemotherapy again knocked the cancer back to undetectable levels. Doctors also used chemicals and radiation to obliterate his bone marrow, the source of all his blood cells—a crude but effective strategy to destroy any cancerous ones that survived chemotherapy. Then they infused him with a bone marrow transplant from his brother. Stem cells from his brother's marrow reconstituted his blood cells, including immune system cells, and within a year, Wartman was working full time and running regularly. "I was almost back to my baseline," he says, as his runs gradually lengthened and his speed improved. But in 2011, "all of a sudden I hit a wall." Leukemia had returned, and his prognosis was bleak. "The outcomes with patients who relapse a second time with ALL are abysmal," he says.
Chemotherapy this time did nothing for his leukemia and almost killed him. But then a cutting-edge technique came to the rescue. A genetic analysis performed at WUSM's genome center, where Wartman worked, pinpointed a hyperactive gene, and a drug for advanced kidney cancer happened to inhibit the protein that gene produces. He went into remission for the third time. A front-page story in the 8 July 2012 issue of The New York Times on the promise of targeted cancer treatments highlighted the success. "While no one can say that Dr. Wartman is cured, after facing certain death last fall, he is alive and doing well," the story said.
Concerned that the drug by itself might not keep his aggressive cancer at bay, Wartman opted for a second transplant—this time with stem cells isolated from peripheral blood from an unrelated donor. He steadily regained his health and returned to seeing patients and working on leukemia experiments in mice. Daily walks with his two dogs again became part of his routine, as did a 6.5-kilometer Saturday run.
Today, Wartman, now 39, remains in remission, but in the cruelest twist of all, he's struggling to survive the cure.
A dearth of options
Sitting barefoot in the living room of the immaculately restored 1897 brick home he shares with his partner, Wartman unfolds a pair of socks. "All right, let's see if I can put these on," he says. He slides one of them onto a curved piece of plastic called a Sock Aid that has two ropes attached, a device octogenarians commonly use. He slowly wiggles a sock onto each foot. He then lifts one leg and moves it atop a Nike running shoe. "I can touch my ankle, but I can't reach my toes," he says, wedging his foot into the shoe with the help of a half-meter-long shoehorn.
Wartman has a chronic form of graft-versus-host disease (GVHD), a debilitating consequence of blood stem cell transplants. It occurs when immune system cells from the donor proliferate and attack the host's tissues. That immune attack inflamed and weakened Wartman's muscles so much that a fall in January cracked his skull. Necrosis of the blood vessels that feed his hips have caused one to collapse, leaving one leg several centimeters shorter, and he has a slow, limping gait. His eyes are so dry that he must put in drops every 20 minutes, and he has painful sores in his mouth. His skin has become leathery in some places. So far, GVHD has spared his organs, but it could damage his liver, lungs, gastrointestinal tract, and genitals. The steroids he takes to calm his transplanted immune system have bloated his face and put him at high risk of infection and osteoporosis.

Wartman in his lab in March 2011 (left), before he developed chronic graft-versus-host disease, and last month at a physical therapy session (right).
PHOTOS: (LEFT TO RIGHT) LUKAS WARTMAN; WHITNEY CURTIS
Wartman's condition is severe but not unusual. GVHD affects up to half of the more than 30,000 people worldwide each year who receive an immune system transplanted from a donor, as either bone marrow or peripheral blood stem cells. The number of transplants—and cases of GVHD—are increasing, yet treatments have not kept pace. Steven Pavletic, who heads the Graft-versus-Host and Autoimmunity Section at the National Cancer Institute in Bethesda, Maryland, says the standard treatments—corticosteroids such as prednisone—"carpet bomb" the immune system, causing a host of side effects and weakening the immune response to potentially deadly infections.
Major recent advances in understanding GVHD's underlying biology, improved animal models, better targeting of interventions, and more systematic clinical trials are finally moving beyond "accumulated sketchy information," Pavletic says. Still, no GVHD treatment has yet received regulatory approval, and with a limited market and a long history of failed clinical trials, drugmakers are leery of getting involved.
The urgency of his case has turned Wartman into one of the world's few patients who advocate for GVHD research, prevention, and treatment. "Most people it affects suffer quietly," says Wartman, who has written journal articles and given presentations and media interviews about his condition. "They're grateful they're alive, and they're beaten down. It's the paradox of being cured and dying of the cure. Even if you can get past that, you don't have the energy to advocate, and that's really tragic."
Immunologic mayhem
Cell transplantation to treat leukemias began with bone marrow transplants in the 1950s and '60s. But those early attempts routinely failed because, unless the donor was an identical twin, the host's immune system attacked the foreign stem cells before they became established. Advances in immunology in the late '60s improved the ability to identify closely matched siblings who were not twins, expanding the pool of potential donors. That, coupled with improved management of GVHD and other complications, led success rates to climb dramatically by the mid-1970s.
More recently, physicians have learned how to harvest blood-forming stem cells from a donor's blood and transplant them into a patient, sidestepping the need for an operating room and a needle stick into the pelvis to extract bone marrow. New immunosuppressive drugs also have made it possible to transplant bone marrow or peripheral stem cells from a donor less closely matched to the host. And changes to the conditioning regimens that wipe out the host's cells have extended transplants to leukemic patients older than 55, who previously were excluded because they couldn't handle the toxic effects.
As a result, cell transplants have become more common and more successful. The Worldwide Network for Blood & Marrow Transplantation has documented a 57% jump in using cell transplants to treat otherwise highly lethal blood cancers from 2006 to 2012. Up to half of the cancer patients with intermediate level disease who received transplants survived longer than 3 years. Some 70% of transplants are now done with peripheral blood, even though that approach has a slightly higher risk of triggering GVHD. All told, those trends have increased the incidence of this medically created condition, which kills 15% to 20% of people who develop it.
GVHD can occur in two stages. Within 100 days of the transplant, up to half of patients develop an "angry" skin rash, nausea, vomiting, diarrhea, and liver abnormalities. That acute phase begins with the conditioning regimen, which damages tissue, leading to a storm of immune messengers called cytokines; trauma to the gut causes extra problems as it leaks bacteria into the bloodstream. In response, the graft's T cells, the pit bulls of the immune system, go haywire. The cytokines speed up T cell cloning and knock down regulatory T cells, which put the brakes on the cloning process. Antigen-presenting cells add to the mayhem by displaying pieces of host antigens and bacteria from the gut to the T cells, further driving their expansion.
Acute GVHD is "entirely a T cell–mediated disease, most people would agree," says Paul Martin, an oncologist and transplant veteran at the Fred Hutchinson Cancer Research Center (the Hutch) in Seattle, Washington. Those battalions of cells bombard tissues that make up the gut, liver, and skin, attacking them as "foreign."
In about one-third of cases, immunosuppressive treatment resolves the acute phase of GVHD within 45 days, while the body's normal checks and balances eliminate the T cells doing the damage, leaving populations that "tolerate" the new host. That's what happened with Wartman when he received his brother's bone marrow. But, as occurred with his second transplant, acute GVHD symptoms can morph into the chronic form of the disease. (About one-third of people who develop chronic GVHD never go through an acute phase.)
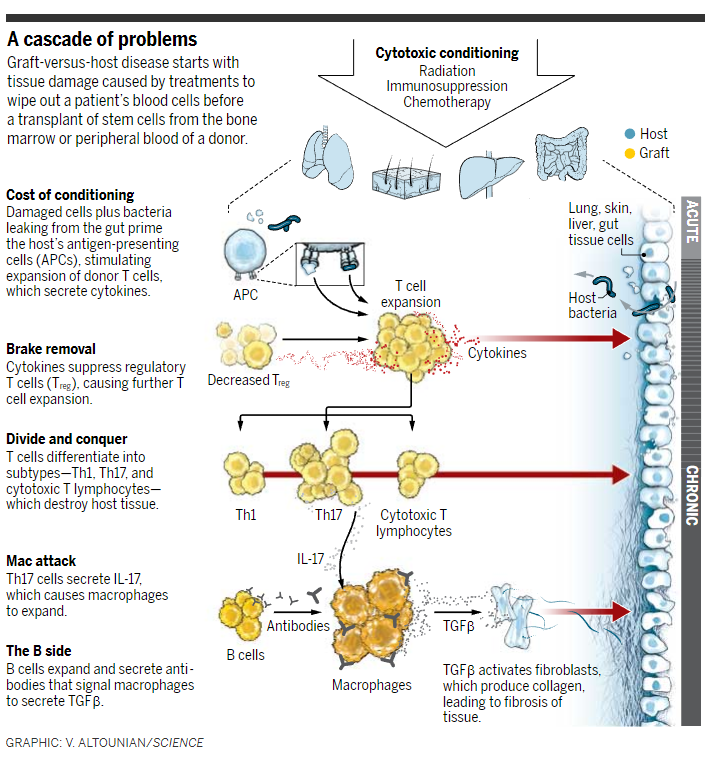
The biology of chronic GVHD remains murky. On top of the T cell abnormalities, chronic GVHD involves antibody-producing B cells and scavenger cells called macrophages. Accumulating evidence suggests that, like T cells, donor B cells are over-produced, pumping out high levels of antibodies that can attack the body's own tissues. Separately, the high levels of a type of T cell called Th17 cells secrete gobs of interleukin-17 (IL-17), expanding macrophage populations. The antibodies also make matters worse by attaching to receptors on the macrophages, which in response spit out transforming growth factor β, an immune messenger that activates collagen-producing fibroblasts, leading to fibrosis of tissues.
Researchers trying to tamp down GVHD have to deal with a major complication: Some graft-versus-host response is good. Convincing evidence for that came in 1979 from a team at the Hutch led by E. Donnall Thomas, who later won the Nobel Prize in Physiology or Medicine for his pioneering work. His Hutch team found that leukemia patients who received bone marrow transplants from an identical twin had far higher relapse rates than people who used a closely matched, but not identical, sibling. The reason is that some cancer cells remain even after the strongest conditioning, and the graft's attack on the host can mop up the stragglers.
The need to preserve some graft-versus-leukemia (GVL) effect has hampered attempts to fight chronic GVHD. "If you're aggressive against the complication, you compromise the therapeutic effect of the transplant," Pavletic says. "That's been the core challenge from the beginning, and it's still a core challenge today."
Struggling for balance
Along with steroids, the mainstay of GVHD treatment today is drugs that turn down the production of IL-2, a cytokine that helps T cell populations expand and diversify. Wartman stabilized his GVHD with high doses of prednisone and the IL-2–dampening drug tacrolimus, but that wasn't enough suppression to resolve his symptoms. And because GVL depends on T cells, oncologists hesitate to suppress them completely. "There's a price to pay if you prevent too much," says Leo Luznik, an oncologist and hematologist at Johns Hopkins University School of Medicine in Baltimore, Maryland, "and unfortunately we don't have a thermostat that we can turn up or down" to get the T cell suppression right.
Luznik and colleagues have sought the right balance by giving high doses of cyclophosphamide, a chemotherapy drug with immunosuppressive effects, shortly after transplantation. That approach, which shuts down the hyperactive donor T cells when they first arrive, has not only allowed patients to tolerate grafts from increasingly mismatched hosts but, in several recent studies, has also cut rates of severe acute and chronic GVHD to less than 15%. Although some researchers worry about increased risk of relapse, the treatment has steadily won over skeptics.
Another approach to taming the donor graft relies on a mixture of anti–T cell anti-bodies called antithymocyte globulin, which is produced in horses or rabbits. Given to the patient shortly before the transplant, the infusion of antibodies theoretically reduces the host's residual T cells, minimizing the risk of graft rejection while eliminating T cells from the donor to thwart GVHD. But the approach is controversial. "For every study where there's a benefit, there's a study where there's no benefit," says pediatric oncologist James Ferrara, who studies GVHD at the Icahn School of Medicine at Mount Sinai in New York City.
A more targeted approach depletes a specific cell population in the graft: naïve T cells, which have not yet differentiated into specific types. A team led by the Hutch's Marie Bleakley and by Warren Shlomchik of the University of Pittsburgh School of Medicine in Pennsylvania removes naïve T cells from grafts with a magnetic system that uses monoclonal antibodies bound to iron beads. In a 2015 report, they said that although the approach had no impact on acute GVHD in 35 patients who received peripheral blood stem cell transplants, it sharply reduced the risk of the disease's chronic form. Relapse rates were unchanged. "That's definitely a step forward," says Geoffrey Hill, a transplant hematologist and researcher at Royal Brisbane and Women's Hospital in Australia.
Hill's lab has focused on a different bad actor: overproduction of IL-6, which causes Th17 cells to proliferate. Hill and colleagues treated 48 transplant patients with tocilizumab, a monoclonal antibody approved to treat rheumatoid arthritis that blocks the IL-6 receptor on cell surfaces. Only 12% developed acute disease, they reported in 2014, although the intervention had no discernible impact on rates of chronic GVHD. A second independent group reported similar results, and animal experiments suggest that the treatment has no impact on the GVL effect. Neither tocilizumab nor naïve T cell depletion has yet proved its worth in randomized studies, however.
And in work that has not yet reached the clinic, the Hutch's Leslie Kean and her team built on their discovery that patients with GVHD have high levels of a molecule called OX40 on the surface of T cells. In experiments on monkeys, they combined an antibody to OX40 with the drug rapamycin, which slows production of IL-2, and saw "total control" of GVHD: Five of five animals had no signs of the disease 100 days after a graft from a highly mismatched donor.
Soon, Ferrara says, better ways to identify who is most likely to develop GVHD will markedly improve options for people who receive cell transplants. In search of early warning signs of the disease, he and co-workers studied almost 700 transplant recipients, comparing protein levels in the blood of patients who developed GVHD with those who did not. This year, his team showed that patients with elevated levels of proteins known as ST2 and REG3α were more likely to suffer severe GVHD and die. Such biomarkers, he says, could eliminate use of prednisone, a risky steroid, in patients not likely to develop GVHD and could signal the need for aggressive treatments in people at high risk. More important, the biomarkers could help researchers choose the best participants for clinical trials.
Promising possibilities
Until some of those treatments prove themselves, Wartman and other patients with chronic GVHD face an endless series of drugs that are approved for other conditions or still stuck in experimental limbo. Wartman, in consultation with his primary doctor, WUSM hematologist and oncologist John DiPersio, has tried many unproven interventions and is still searching for others. "It's a challenge taking care of him because he insists on doing stuff his own way," DiPersio says. "But he's one of the smartest guys around. And it's been painful for me to see him get weaker over the past 5 years. It has been slow but inexorable. It's profoundly annoying not to be able to come up with some brilliant approach to turn things around for this guy."
Wartman tried the voodoo-sounding extracorporeal photopheresis, which involved implanting a catheter in his chest to run blood twice a week through a machine that filtered out white blood cells, killed them with chemicals and ultraviolet light, and then returned the blood to his circulation. Although that procedure relieved some of his chronic GVHD symptoms, the benefits waned and he stopped after about 60 treatments. The rheumatoid arthritis drug etanercept, which inhibits a key cytokine that leads to inflammation, also had no effect.
In July 2015, his muscles wasting away, Wartman joined a clinical trial of a drug, ibrutinib, approved to treat specific blood cancers. The drug inhibits a tyrosine kinase enzyme that helps antibody-producing B cells mature, and in the trial it seemed to help many participants. But not Wartman. "He initially tolerated it well and it seemed like he was having a response, but after about 3 months, he had an adverse reaction to the drug and he just wanted to stop it," says Iskra Pusic, a GVHD specialist at WUSM who helps with his care.
After that, Wartman began taking the drug ruxolitinib, which is on the market to treat a bone marrow disorder that leads to anemia. (The drug costs about $10,000 per month, and he had to appeal to his insurance company to cover its off-label use.) It inhibits two tyrosine kinase enzymes called Jak1 and Jak2, which rev up production of immune cells. Researchers in DiPersio's lab showed in a mouse model that the drug can reduce GVHD without compromising the GVL effect. Wartman continues to take ruxolitinib to this day in combination with steroids.
Last year, the U.S. Food and Drug Administration recognized the promise of both drugs, designating them "breakthrough therapies"—ibrutinib for chronic GVHD and ruxolitinib for the acute disease. (Clinical data don't yet exist for its use in chronic cases like Wartman's.) Those drugs may soon become the first approved treatments for GVHD.
With Wartman's GVHD in a holding pattern, he keeps searching for other drugs to try off-label or in clinical trials. "Unless we find something to take my inflammation down a notch, things will just continue to progress," Wartman says. He remains optimistic that even if a new drug cannot undo the damage from GVHD, it will improve his quality of life. "I may be able to get back to doing some of things I'm not able to do now," he says, such as simply walking his dogs.
Neither DiPersio nor Wartman regrets the decision to undergo a stem cell transplant, accepting the chronic GVHD as the tradeoff for an increased GVL effect. "My leukemia couldn't have been cured unless this immunological onslaught was allowed to occur," Wartman says.
He adds that he is grateful his professional connection to the disease has given him the best options for dealing with the devastating consequences of his cure. "I take care of patients who are going through terrible, terrible complications who deal with them without any recourse," Wartman says. "These are people I often consider have lives that are more worthwhile than mine. I've taken care of so many patients in their early 20s to early 40s who have productive careers in the workforce or are raising children, and their lives are cut short not by the disease but by the cure. As a group, transplant physicians feel they're doing something to save people's lives and underestimate the impact of a transplant. But I don't."
Sourche:
Posted in: HealthCancer topic
DOI: 10.1126/science.aan7079
http://www.sciencemag.org/news/2017/07/stem-cell-transplant-helped-beat-back-young-doctor-s-cancer-now-it-s-assaulting-his
Hi! I am a robot. I just upvoted you! I found similar content that readers might be interested in:
http://www.mantouchong.com/2017/07/a-stem-cell-transplant-helped-beat-back-a-young-doctors-cancer-now-its-assaulting-his-body/