Elements, Compounds and Mixtures – An element is chemically the simplest substance and hence cannot be broken do using chemical reactions.
A chemical element or element is a species of atoms having the same number of protons in their atomic nuclei (i.e. the same atomic number, or Z). There are 118 elements that have been identified, of which the first 94 occur naturally on Earth with the remaining 24 being synthetic elements. There are 80 elements that have at least one stable isotope and 38 that have exclusively radioactive isotopes, which decay over time into other elements. Iron is the most abundant element (by mass) making up Earth, while oxygen is the most common element in the Earth's crust.
In chemistry, a mixture is a material made up of two or more different substances which are mixed but are not combined chemically. A mixture refers to the physical combination of two or more substances in which the identities are retained and are mixed in the form of solutions, suspensions, and colloids.
A chemical compound (or just compound if used in the context of chemistry) is an entity consisting of two or more atoms, commonly two from different chemical elements, which associate via chemical bonds. There are four types of compounds, depending on how the constituent atoms are held together: molecules held together by covalent bonds, ionic compounds held together by ionic bonds, intermetallic compounds held together by metallic bonds, and certain complexes held together by coordinate covalent bonds. Many chemical compounds have a unique numerical identifier assigned by the Chemical Abstracts Service (CAS): its CAS number.
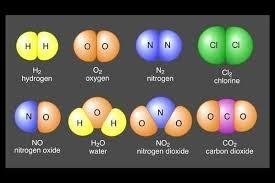
The Rules in Writing an Element:
1. The Alchemy Period

In the alchemy period, elements and compounds the had not been understood, so it was possible that the elements and compounds had the same symbols. For example, water have the same symbol as that of iron.
2. Based on John Dalton’s Theory

The summary of John Dalton theory (a British scientist) is shown in the following statements.
- An atom is the smallest part of matter and indivisible particle
- All atoms of a particular element are identical
- Different elements have different atoms.
- Atoms combine in certain whole-number rations.
- n a chemical reaction, atoms are merely rearranged to form new compounds, they are not created, destroyed or changed into atoms of any other elements.
3. Based on Berzelius Theory

Jons Jacob Berzelius (a Swedish chemist) wrote the symbols of elements by using letters known as an elemental formula. The later rule becomes the base of the official rule in writing the symbols of elements of IUPAC (International Union of Pure and Applied Chemistry). The elements in the universe can be classified into metals and non-metals, shown in the following Periodic Table.
A compounds is a substance that is composed of two or more elements with a specific ratio ( a fixed ratio) and the properties of its constituents can’t be seen anymore. Based on the elements which form compounds, compounds can be classified into two categories. They are : ## 1. Organic compounds Their constituents are non-metal elements, usually carbon, hydrogen, sometimes oxygen and nitrogen. Example : a. The components of crude oil, such as methane, ethane b. Alcohols, such as methanol, ethanol.
2. Inorganic compounds
An inorganic compound is made up of :
a. A reaction between metal elements and non-metal elements
b. A reaction between hydrogen and non-metal elements or halogen
c. A reaction between oxygen and non-metal elemen
Mixtures can be classified into 2 categories. They are :
1. Homogeneous mixtures
Homogeneous mixture is a mixture whose constituents can’t be seen anymore.
a. Solution, such as : sugar-water solution, milk, salt-water solution
b. Alloy (the combination of metals), such as stainless steel and the combination of iron.
2. Heterogeneous mixtures
A heterogeneous mixture is a mixture whose constituents still exist. Example : soil, lime-water solutions.
Separations of Mixtures
1. Chromatography
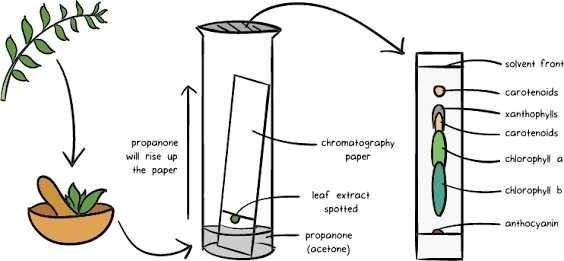
Chromatography is used to separate chemical substances that have different rates of spreading in a specific solvent. Example : the separation of two colors so we can know the components of each color.
2. Filtration

Filtration is the separation of mixture based on the differences of particles size in it. Example : the separation between grated coconuts and water to produce coconut milk.
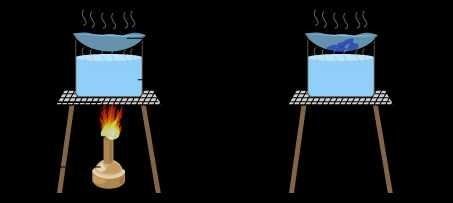
3. Distillation

Distillation is the separation of mixtures based on their differences in boiling points. Usually the mixtures that will be separated are liquid. Example : The separation pure water from sea water.
4. Evaporation
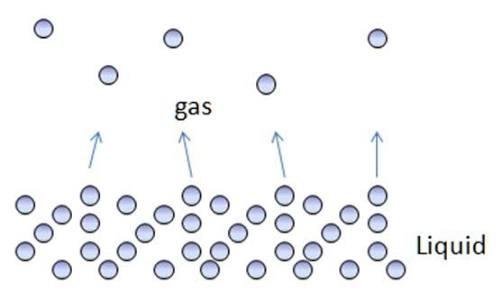
Evaporation is the separation of mixtures based on the difference in the vaporizing of substances in the mixtures. The mixtures that will be separated are in liquids.
5. Crystallization
The principle of cystallization is the same as that of evaporation. The substances that can’t evaporated will be retained in the bottom of the container as crystals.
6. Extraction
Extraction is the separation of mixtures based on their difference in solubility caused by difference in density.
7. Sublimation

Sublimation is the separation of mixtures based on their properties. Example : The purification of dirty naphthalene.
Sources :
Copying/Pasting full texts without adding anything original is frowned upon by the community.
Some tips to share content and add value:
Repeated copy/paste posts could be considered spam. Spam is discouraged by the community, and may result in action from the cheetah bot.
If you are actually the original author, please do reply to let us know!
Thank You!