Hello all steemians, this is the first topic I want to share you about science worksheet. I hope its useful for us and for science lover or science teachers.
Here I will share about Science Worksheet : Solar Power Supply Solution.
Solar power is the conversion of energy from sunlight into electricity, either directly using photovoltaics (PV), indirectly using concentrated solar power, or a combination.Source
Based on their electrical conductivity, the solution can be divided into electrolyte and non-electrolyte solutions. Electrolyte solution is a solution that can conduct electrical current. While the non-electrolyte solution is a solution that can not conduct electrical current.
The ability of a solution to conduct an electric current depends on the presence of dissolved ions in the solution. The more dissolved ions, the greater the capacity of the solution. If in the solution there are no dissolved ions in it, then the solution can not conduct an electric current.
Tools and Materials
Tools:
- Battery 1.5 V
- Small bulb
- Masking tape
- Nails
- Scissors
- Glass
Ingredients:
- Dishes
- Orange water
- Salt kitchen
- Kerosene
- Soy sauce
- Syrup
- Coconut water
- Tissue
Procedure
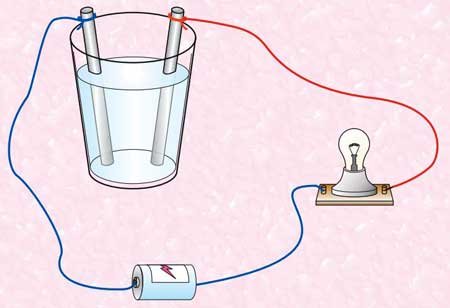
- Complete the tool as shown above.
- Wash nails (electrode) then dry with a tissue.
- Put the water into a glass.
- Insert the electrode into the solution. Keep the electrodes close together but not touch.
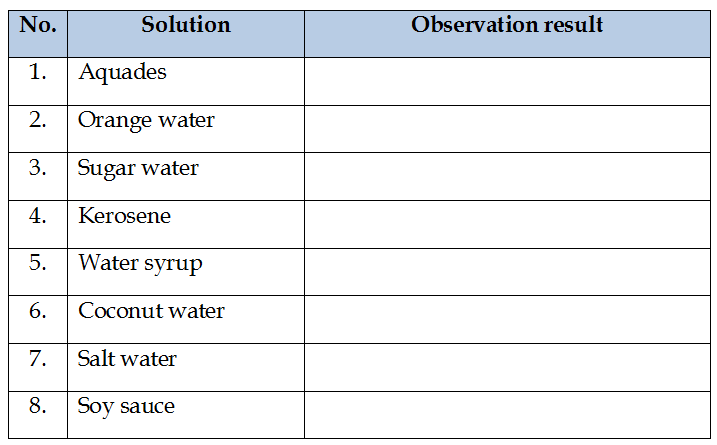
- Record the observations on the sheet provided.
- Perform steps 2-5 for other solutions.
Observation Data
Question
- Of the many solutions, which one can conduct an electric current? Why are these solutions able to conduct an electric current?
- Of the many solutions, which one can not conduct an electric current? Why are not these solutions able to conduct an electric current?
- Which solution has the greatest electrical conductivity? Explain!
- Make a report of the results of the experiment and present the results in front of the class!
Conclusion
...............................................................................................................................................................................................................................................................................................................................................................................................................................................