Hello Dear steemian, today i will share you a post, that something can you do with backing soda, sometime a girl/mother uses backing soda to make a bread or cake, but you can also make a magic crystal from this material. Well, lets to the point
 Source :pixabay cc
Source :pixabay cc
When someone hearing the word about "crystal", someone would imagine it is a diamond or a kind of it, or some people would think if the crystallization process occurs only in a crystalizer or refrigerator (water to ice). Have you ever imagined that crystallization process can also form in open space or room temperature? Lets we learn about crystallization.
What Does Crystal Mean ?
 Source :rahasiaa
Source :rahasiaa
Crystallization is a change of a liquid phase into a solid or solid formation from solution precipitation, Thermodynamics and cooling profiles may affect the Crystallization process, for example, a heating of molecular in the crystal will damage the molecular structure and out of its position (form). The process of crystal forming at room temperature can we see an example such as "Hot Ice". Hot ice is not really an ice but a nickname for Sodium Acetate. Sodium acetate is a chemical compound composed of salts and acids, sodium acetate has chemical formula CH3COONa, if we separate this formula then it look like CH3COO- (acid) and Na+ (Salt). So, The forming of Sodium acetate we can react some elements or compound to form sodium acetate, such as, baking soda (NaHCO3), vinegar (CH3COOH) and sodium hydroxide (NaOH). Chemically, the equation can be formulate as follow :
Reaction baking soda and vinegar
CH3COOH + NaHCO3 → CH3COONa + H2CO3
Advance reaction
H2CO3 → CO2 + H2O
Overall reaction can be formulated as follow :
CH3COOH + NaHCO3 → CH3COONa + H3O + CO3
Reaction vinegar and sodium hydroxide
CH3COOH + NaOH → CH3COONa + H3O
In the first reaction, the manufacture of sodium acetate from baking soda and vinegar produces sodium acetate and carbonic acid as a by-product. in the second reaction, the formation of sodium acetate from vinegar and sodium hydroxide produces sodium acetate and H3O as byproducts. Sodium acetate is an acid and salt compound.
So that a chemical compound containing salt (Na) is required to produce Sodium Acetate, Such as Baking Soda (NaHCO3) and Sodium Hydroxide (NaOH), both of these compounds contain salt element in the compound.
How Can it Be ?
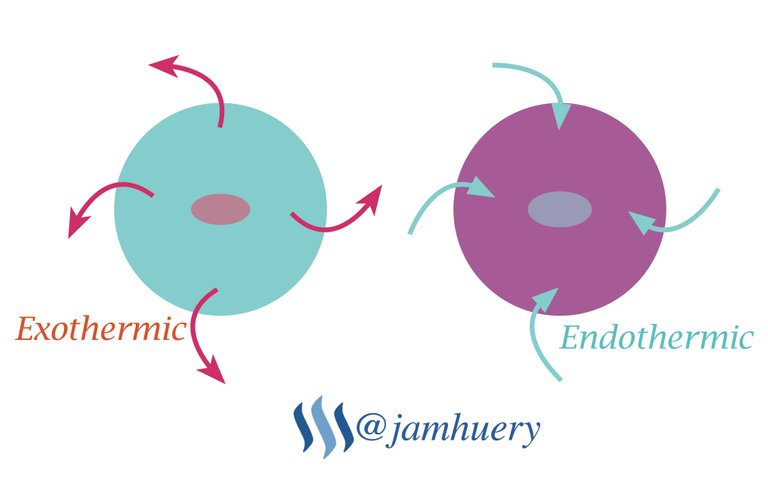
Crystallization is an exothermic process where heat was releases to environment, Therefore, sodium acetate referred as "hot ice", sodium acetate has melting point of 136.4°C, example, by adding ice into solution, so a temperature drop will change it to a crystal, the crystal acts as a nuke site (formation of core grains), usually it occurring at lower temperatures (supercooling), it means, the crystal was more easily damaged than formed it or dissolve a crystal in some solvent is more easily than forming a crystal back from the solution. This phenomenon makes hot ice look like a magic
Conclusion
Conclusion
The formation of crystals in sodium acetate (Hot Ice) or the alteration of the liquid phase into solid phase due to temperature changes, by adding ice in solution will occur exothermic, so the solution of sodium acetate will freezing or become to crystal.
Source :
Support Scientist By Use #science tag or join @steemSTEM
Follow Me @jamhuery

Magic! ♡
thanks for visting @missthreedom
wonderful I will try. please follow me
Peace, Abundance, and Liberty Network (PALnet) Discord Channel. It's a completely public and open space to all members of the Steemit community who voluntarily choose to be there.Congratulations! This post has been upvoted from the communal account, @minnowsupport, by jamhuery from the Minnow Support Project. It's a witness project run by aggroed, ausbitbank, teamsteem, theprophet0, someguy123, neoxian, followbtcnews/crimsonclad, and netuoso. The goal is to help Steemit grow by supporting Minnows and creating a social network. Please find us in the
Good post my friend.. @jamhuery
GOOD magic idea for school children also very nice very clear explanation of the process.