Basically All Gems are Pure Silica (Al2SiO4)O
Silica is the name given to a group of minerals consisting of silicon and oxygen. These two elements are most abundant in the earth's crust. Silica is found generally in crystalline form and rarely in an amorphous state. This is because silica consists of bonding one silicon atom and two oxygen atoms, the chemical formula of silica is SiO2.
Sand consists of granules or small particles of minerals and rock fragments. Although granules may be derived from any mineral composition, the main component of the sand is usually a quartz mineral consisting of silica (silicon dioxide). Other components that may be present in the sand include aluminum.

The Reaction Process at Hydrothermal Reaction.
The hydrothermal system is defined as the hot fluid circulation (500 -1200 ° C), laterally and vertically at varying temperatures and pressures below the earth's surface. This system contains two main components, namely heat source and fluid phase.
The circulation of the hydrothermal fluid causes the mineral set on the wall rock to become unstable and tends to adjust the new equilibrium by forming a set of minerals corresponding to the new conditions, known as hydrothermal alteration. Hydrothermal mineral deposits may be formed due to leaching of the hydrothermal fluid, transport, and precipitate new minerals in response to both physical and chemical changes.
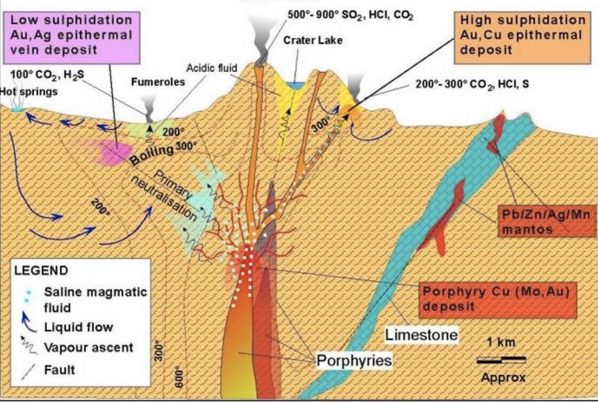
Illustration Of Reaction Process
Example 1: Chemical Compound of Emerald Be3Al2(SiO3)6, the Color Dark Green.
Emerald is the most prized assortment of the mineral Beryl. It now and then brings higher costs than precious stone. It shows up as light green to splendid green. The run of the mill shade of emerald is a lovely, unmistakable tint known as "emerald green" and is because of hints of chromium, once in a while vanadium, in the gem structure. Emeralds can be light or dull green, splendid green or leaf-green. The shading is exceptionally steady against light and warm, and just changes at 700-800 C. All emeralds are fragile and consolidated with interior anxieties, are delicate to weight; mind must be taken in warming them.
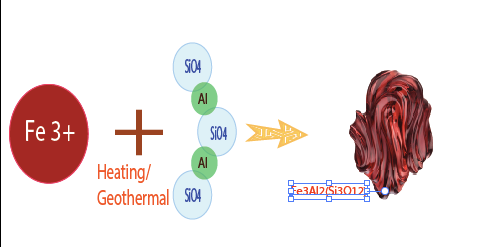
Example : Chemical Compound of Pyrop Mg3Al2(SiO4)3, the Color Light Red. Pyrope is the iron magnesium and aluminum silicate of the pyrope-almandine arrangement in the Pyralspite gathering of the Garnet family. Its wonderful dark red pearl quality makes it a standout amongst the most mainstream. Unadulterated pyrope is drab, yet its red shading, some of the time splendid, is because of little amounts of chrome in the precious stone structure. It shows up as dodecahedral or trapezohedral precious stones, dim red, normally extremely very much framed. It likewise happens as adjusted grains. As segregated, granular precious stones, it regularly shows up as an immaculate rhombic dodecahedron. The shading is frequently rosy dark colored, yet can be an unmistakable red, light red, violet red, or profound blackish red. The precious stones, which are regularly semiopaque, can be straightforward and limpid, with profoundly radiant face.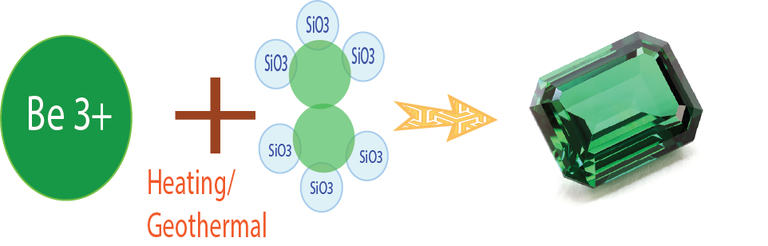
Follow me @jamhuery

Great post ... keep doing your good work!
Great post Jam! I like to see more science on Steemit
Mee too, like to follow you to read about science, thanks @timsaid
Hoah, its so coll information bro. I like it
Thanks, i will update more letter @foways
Good job
Great post, good science and nice reading
nyan sang aqua marine gure ka foto atra jameun nyoeh.....@jamhuery
wooow exelent
Awesome post!
Thanks @hagbardceline
Nice articles @jamhuery, very useful for us
Thanks, zamrud/jamrud/emrald, and Garnet almandine
Great post, I love getting a daily dose of unique science. Followed so keep 'em coming!
Thanks @hansikhouse
아주 좋은 포스트
나를 @kamus 따라
@things, CHEMISTRY!