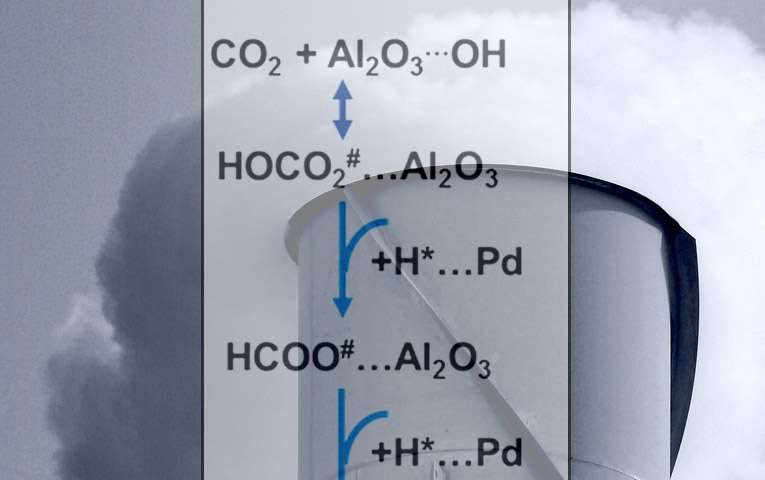
What if we could turn carbon dioxide, CO2, into a valuable resource? Using CO2 as a feedstock to create fuels or other chemicals would offer economic and environmental benefits. The challenge is designing effective processes that yield only the desired chemical: methane or carbon monoxide. Why? Scientists didn't have a clear understanding of the pivotal steps in the reaction mechanism. Scientists at Pacific Northwest National Laboratory, led by Dr. Janos Szanyi, determined that formate (HCOO-), an oft-overlooked ion, was a critical intermediate in the overall CO2 conversion process. The balance in the rates of formate and carbon monoxide intermediates conversion determines what chemicals are produced.
"This study gives us crucial information to use an easily available raw material, CO2, and turn it into something useful—a chemical intermediate, carbon monoxide, or an energy carrier, methane. This intermediate can be used for the production of higher hydrocarbons, or fuels," said Szanyi.
For years, some considered formate nothing more than a bystander, a molecule that didn't contribute to the reaction. Now, the team has shown that formate is indeed vital. Understanding the steps, and the role of formates, lets scientists design a selective catalyst that pumps out the desired chemicals. Understanding the steps in the reaction gives scientists crucial information to control the reaction. Further, the work ends a long-standing controversy about the role of formates on the catalyst's surface.
In this study, a team from Pacific Northwest National Laboratory determined the factors that control a catalyst's selectivity for CO2 hydrogenation to carbon monoxide or methane. They used a novel combination of operando transmission FTIR and SSITKA (Fourier Transform Infrared Spectroscopy/Steady State Isotopic Transient Kinetic Analysis) experiments.
They determined the behavior of key intermediates (formates and carbon monoxide) on the surface of the catalyst. For carbon monoxide formation, the rate-determining step is the conversion, or reduction, of adsorbed formate at the interface of the palladium metal catalyst and the aluminum oxide support. For methane formation, the rate-determining step (see the green arrows in the figure) is adding hydrogen to the adsorbed carbon monoxide. The balance between the rates of absorbed formate reduction and carbon monoxide methanation governs the catalyst's selectivity. That is, the selectivity revolves around how fast the intermediates on the surface pick up hydrogen, creating either carbon monoxide or methane.
Having determined key aspects of the reaction, the team designed three catalysts with different distributions of palladium. The amount of palladium on the surface, they found, is another vital aspect in selecting the products. The catalyst with the least palladium produced both carbon monoxide and methane, with higher selectivity for carbon monoxide. In contrast, higher than 80 percent selectivity towards methane formation was observed over the catalyst with the highest metal loading.
The team is now modifying the environment around the catalyst's active site to tune its selectivity. This work is aimed at reducing the amount of undesired products released. In addition, the team is developing methods to prepare single atom catalysts to increase the efficiency of the precious metals involved.
Read more at: https://phys.org/news/2017-09-scientists-catalyst-greenhouse-gas-fuel.html#jCp
Original Article - https://phys.org/news/2017-09-scientists-catalyst-greenhouse-gas-fuel.html
i love science and good work my friend 😍
so interesting hey!
Hi! I am a robot. I just upvoted you! I found similar content that readers might be interested in:
https://phys.org/news/2017-09-scientists-catalyst-greenhouse-gas-fuel.html
I love it when I find an article that makes me feel good while I'm reading. It's a beautiful point that flows with the current shift happening in humanity. I believe people want to do better and we are evolving into a more loving society.
you are a good writer..
Congratulations @pvtrickheaton! You have completed some achievement on Steemit and have been rewarded with new badge(s) :
Click on any badge to view your own Board of Honor on SteemitBoard.
For more information about SteemitBoard, click here
If you no longer want to receive notifications, reply to this comment with the word
STOP