I have never seen the following compound referenced in the literature:
Possible names might be tris-methylene ether of hexahydroxybenzene? Trimethlenedioxybenzene? Benzo-tris-dioxolane?
Maybe naming is why I haven't found it, but can it even be synthesized? I don't know of any structures containing benzene rings with six phenolic substituents, so if anyone knows any examples please enlighten me.
It may be that increasing the number of phenolic substituents beyond four tends to favor quinone formation. Biological properties of ubiquinone or Coenzyme Q are convenient examples.
Methylenedioxy groups are common substitutuents on organic phenolic groups in many chemicals derived from plants. Almost every class of alkaloids with 3,4-dihydroxyphenyl groups have a methylene ether counterpart.
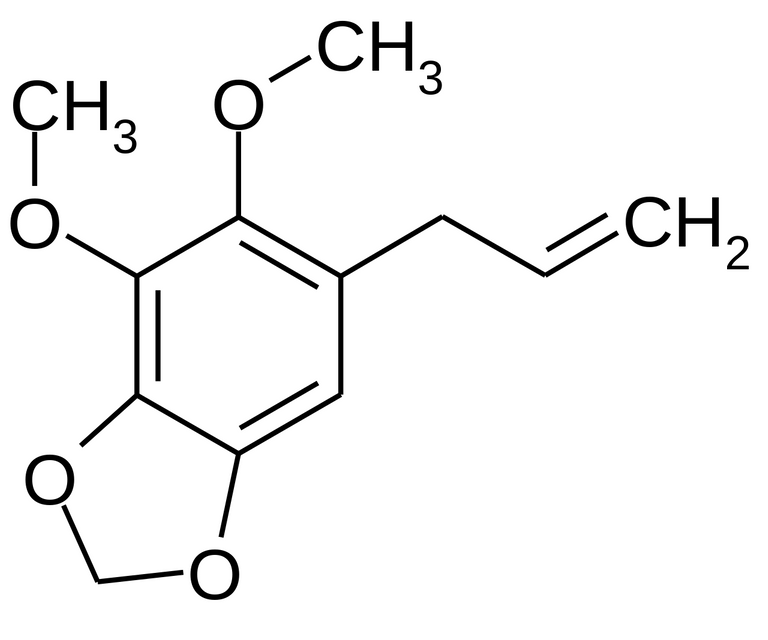
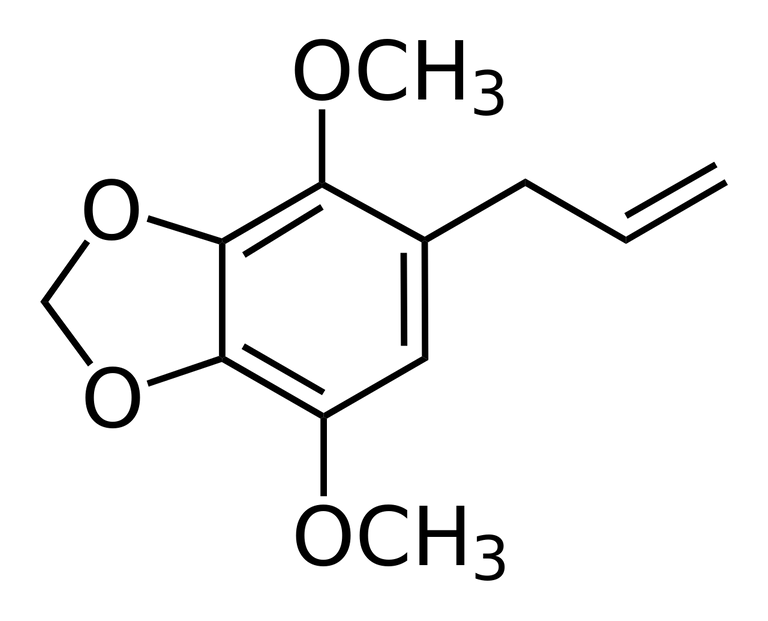
There are examples in nature with as many as four oxygens, as found in apiole from dill and parsley:
In all natural product examples there are either methyl ethers or bridged methylene diethers of adjacent phenolic hydroxyls. It should be possible to synthesize a 3,4- and 5,6-bi-methylenedioxy analog of the apiole, but to my knowledge no one has tried. It could be the precursor to a class of unstudied 3,4,5,6-bi-methylenedioxyphenethylamine analogs.
Hypothetical 3,4-methylenedioxy-4,5-methylenedioxyphenethylamine
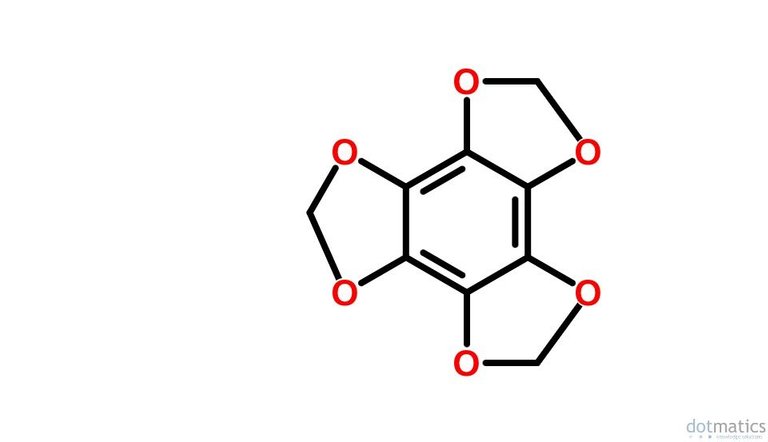
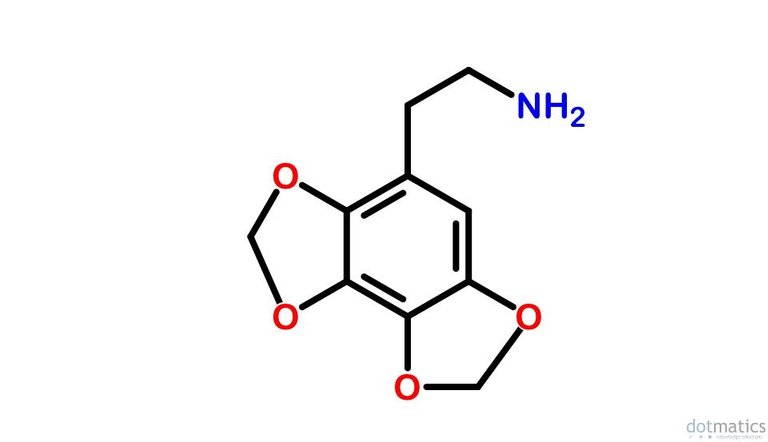
The closest I can come up with to resemble your hypothetical compound is Hexahydroxybenzene triscarbonate - Wikipedia:
Do you think it would be possible to reduce the three -C=O bonds on the outer edges of this stucture to -CH2- bonds to yield the compound that you have postulated?
Certainly! That's a great precursor. And very interesting in its own right.
To the question in your title, my Magic 8-Ball says:
Hi! I'm a bot, and this answer was posted automatically. Check this post out for more information.
Hexahydroxybenzene exists and is also called benzenehexol. It is well studied, known since the early 20th century, forms stable compounds, and has its own Wikipedia entry here.. It is used in liquid crystals and lithium batteries among other things.
Hey it’s @qiyi two years later!
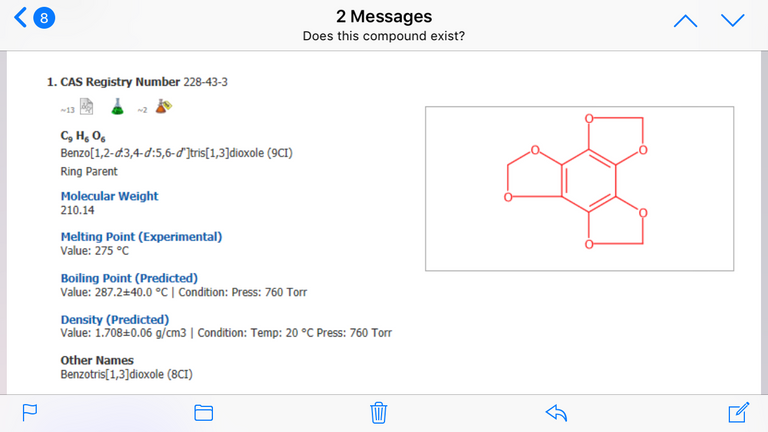
I did find this compound available from Cayman Chemical Co. They said it was patented, but I don’t know for what.
CAS#228-43-3