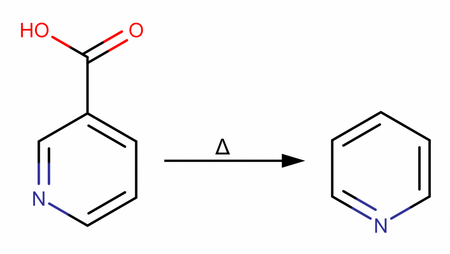
The following is a plain English technique for producing pyridine via the decarboxylation of niacin in the presence of a copper catalyst based on Nile Reds Youtube video. Make sure to familiarize yourself with all steps before attempting any synthesis or process work.
Required Substances & Equipment:
50 Grams Niacin
12 Grams Copper Carbonate
3 Grams Sodium Hydroxides
Simple Distillation Apparatus w/ 2x 250ml Round Bottom Flasks
Stirring Mantle
Ventilation / Chemical Respirator
Amber Bottle
Molecular Sieves
12 G of Copper Carbonate along with 50 G of Niacin are added to a mortar and pestle or food processor and ground until completely homogeneous. It is important that the mixture be evenly blended.
Transfer finely ground and evenly blended Niacin / Copper Carbonate this is what powder to a 250 ml round bottom flask. Prepare your distillation apparatus for traditional distillation, with an additional round bottom flask prepared to receive the distilled Pyridine.
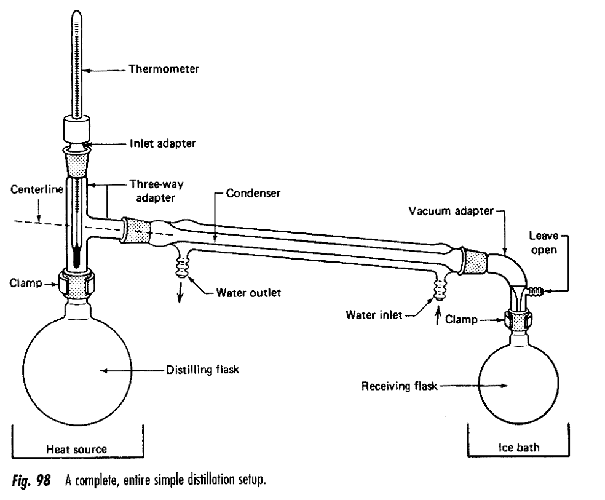
- Place a heating mantle underneath your initial flask containing the Niacin blend and insulate the rest of the flask with foil or otherwise. Begin to slowly heat the powder, make sure you do not exceed 250° C my many degrees, niacin boils at 115° C. Progress will begins slowly, and complete distillation will take place over a scale of hours. The initial distillate recovered will be mostly Water or a Water / Pyridine blend. (Pyridine has off-putting and is toxic, you will require ventilation, and a respirator if you can smell it at all.)
Note: white fume is an indicator the optimal distillation temperature have been exceeded, so lower your temperature if you see it. If the distillation is too slow, insulate the distillation head.
Eventually distillation will slow to a stop and your receiving flask will contain an impure blend of Pyridine and Water. When no more distillate is recovered, separate the receiving flask from the apparatus, add 1 G of Sodium Hydroxide (Lye) to the solution and stirrer to react. The remaining Niacin should fall out of solution and it may form an aqueous layer. Separate using separatory funnel, vacuum filter or both if required. Discard your water layer if one has separated.
To the filtered Pyridine solution, add another 2 G of Sodium Hydroxide to react any remaining Niacin and to pull out any remaining Water. Prepare your distillation apparatus with a fresh receiving flask and heat your Pyridine solution to 115° C. Distillation should be a bit smoother this time and leave a white slurry behind in your distilling flask.
Store in amber glass with a couple grams of molecular sieves for dryness and safekeeping.
Thank you for reading!
NileReds Pyridine video: https://m.youtube.com/watch?v=r-xt24lBbrs