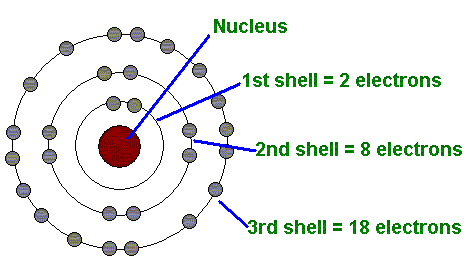
 For modeling an electrical phenomena, we should recall knowledge from elementary physics in memory. The substance is composed of molecules, and each molecule is made up of atoms that are built from the core and the electronic envelope.
For modeling an electrical phenomena, we should recall knowledge from elementary physics in memory. The substance is composed of molecules, and each molecule is made up of atoms that are built from the core and the electronic envelope.
Atoms of substances differ in their size by number of protons and neutrons in the core and by the number of electrons that circulate around it. The hydrogen atom is the smallest one, represented by one proton in the core and one electron in the envelope. All other atoms have several electrons and protons (eg. Cu has 29 electrons).
The electron is elementary particle of the atom by agreement with negative charge (-) and the proton in the core is positive (+). Neutrons, which, just like protons, are located in the core, are electric neutral particles that hold the mass balance equilibrium. Thus, the entire mass of the atom represents the core, since the electrons are pure electric particles. (me = 0,001 mp)
The electricity of charge can always be only a multiple of basic charge of the electron, which is e0 = 1,6 ∙ 10-19 C (As Ampersecond;
Source: http: //en.wikipedia.org/wiki/Coulomb)
For the separation of electrons from atoms we have to invest some kind energy. Invested energy can be returned in the form of electric energy, which is proportional to the quantity of separated electrons and the resulting electrical voltage.
The electrical voltage of 1 Volt is obtained by investing 1 Joule of energy to separate 1 Coulon (1C = 1 As) electric charge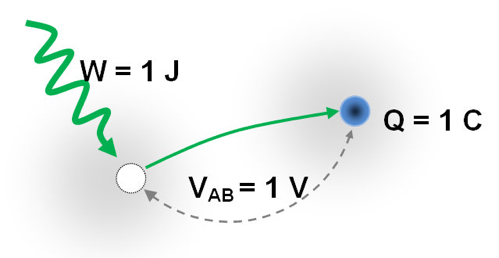 !
!
source of Bohrs Atom Model Picture: https://the-history-of-the-atom.wikispaces.com/Niels+Bohr
Congratulations @stane! You received a personal award!
Click here to view your Board
Congratulations @stane! You received a personal award!
You can view your badges on your Steem Board and compare to others on the Steem Ranking
Vote for @Steemitboard as a witness to get one more award and increased upvotes!