How can we minimize the amount of CO2 in the atmosphere? Maybe clean fuels production is the answer.

Since the announcement of the Kyoto Protocol, most countries have committed to reduce CO2 emissions to the atmosphere and thus reduce the impact on global warming. Therefore, the production of renewable synthetic fuels as an alternative to petroleum fuels is an area that has attracted the attention of various research groups.
Methane, the main component of natural gas, is easily found in nature. It is one of the main greenhouse gases, together with CO2, besides being a very important fuel. The production of synthetic methane has gained relevance because it is considered a clean fuel when compared to others such as coal, as well as high demand and high prices of natural gas [2].
This is why several researches have studied the Sabatier reaction which generates methane using two raw materials that abound in the world, carbon dioxide (CO2) and hydrogen (H2). CO2 is not really a pollutant, since it is essential for life on the planet and as a greenhouse gas it allows to maintain an adequate temperature on earth. However, the burning of fossil fuels generates too much CO2 which results in a greater accumulation of solar heat and causing global warming [3]. Hydrogen is obtained mainly by water electrolysis, and it is the lightest and most abundant element in the universe.
This reaction requires high temperatures to be carried out, so it is necessary to use catalysts that help the process. Among the most used catalysts are those of nickel and ruthenium. Since it uses CO2 as a reactant and produces CH4, this reaction represents a way to reduce the concentration of CO2 in the environment and produce CH4 that serves as fuel.
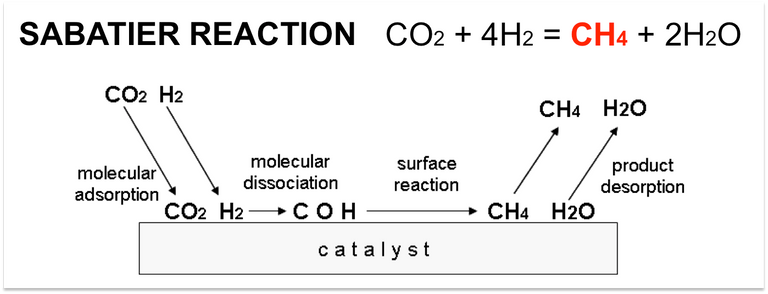
This reaction was discovered by the French chemist Paul Sabatier (Nobel Prize winner in 1912 [5]) in 1910 when he realized that some amorphous metals can serve as catalysts for hydrogenation reactions of organic substrates. [6].
Paul Sabatier[5]
In addition to reducing the concentration of CO2 on the planet this reaction is able to convert space waste into fuel. All the waste (from clothes, leftovers to feces) that are generated in space missions can be fed to the reactor where the CO2 is transformed through Sabatier's reaction into CH4 to be used as fuel in space operations [7]. Also, this reaction serves as a source of water for the crew's consumption [8].
Despite the importance of this reaction, it is still necessary to develop the process on a large scale. Studies of catalysts synthesis have focused on increased production of CH4; however, it is vital to reduce the reaction temperature for the process to be feasible [9]. This means that there is still a long way to go to be able to use this technology efficiently.
References:
1. Carbon dioxide captured from air can be directly converted into methanol fuel
2. Methane production by a combined Sabatier reaction/water electrolysis process
3. The tech race to save Earth from global warming by absorbing carbon from the air
4. ABIOTIC METHANE
5. Paul Sabatier - Biographical
6. Dogma-breaking catalysis
7. TURNING SPACE GARBAGE INTO ROCKET FUEL
8. New ISS machine makes water from waste CO2
9. CO2 Methanation: The Effect of Catalysts and Reaction Conditions

Congratulations @tavorm! You received a personal award!
Click here to view your Board
Congratulations @tavorm! You received a personal award!
You can view your badges on your Steem Board and compare to others on the Steem Ranking
Vote for @Steemitboard as a witness to get one more award and increased upvotes!