The Miracle Material
Andre Geim, a physicist, was a brilliant researcher working for the University of Manchester. Besides working on just one research project, Geim took on many temporary projects. He called them “Friday night experiments.” Our story begins in 2002, when Geim assigned a graduate student one of these “Friday night experiments” to help him get acquainted with the lab. The experiment was simple. Try to get graphite as thin as possible. Geim also worked on the experiment with his colleagues, and in a sudden moment of brilliance, a colleague brought Geim a simple piece of scotch tape that was recently taken from a graphite surface [1]. By 2004, two men, Andre Geim and his closest colleague, Konstantin Novoselov had isolated the flakes on the scotch tape and studied them. They had discovered something truly amazing. They had discovered a material that was only one atom thick (Yes, the thinnest material). This material was also as strong as it was thin. Upon further examination, this material was 200 times the strength of steel and only one-sixteenth as dense. It could bend, stretch, flip, or be folded[2]. Yes, I am talking about graphene.
What is graphene?
Structure
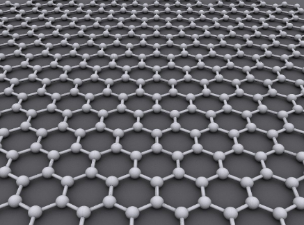
Graphene’s exceptionality is best understood through its crystalline lattices. A crystalline lattice is the natural way the atoms of a crystalline solid aggregate to minimize their energy[1]. You can think of a lattice as a crystal structure. Different lattice structures change the properties of the materials they make up. A few examples of materials with crystalline lattices are quartz, ice, salt, and diamonds. Here is a picture of graphene’s lattice.
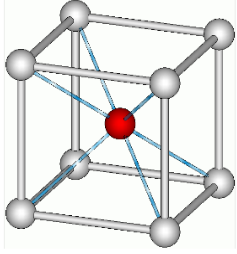
Graphene has a simple 2-dimensional hexagonal lattice structure. Usually, lattice structures are pretty difficult to understand because most are 3-dimensional, but graphene’s is 2-dimensional. Graphene’s 2-dimensional structure is one of the two reasons why graphene is so strong. All the bonds in graphene are on a singular plane. This means that if you were to somehow pull on a graphene nanoribbon, all (or most) the bonds would be working against you[3]. For reference, steel is typically a body centered lattice and diamonds have a special kind of lattice structure called diamond cubic. Here are some pictures.
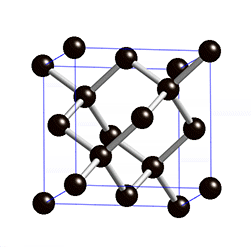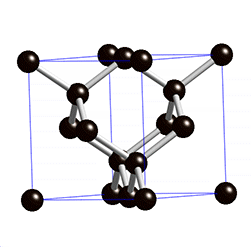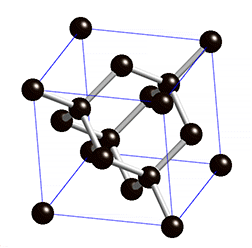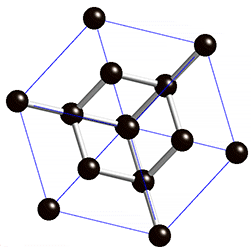
Diamond Cubic
Steel has a greater packing efficiency than graphene (as seen above). An important thing to note is compactness doesn’t necessarily mean strength. However, compactness is directly correlated with density. This is why the open lattice of graphene is one-sixteenth the density of steel.
Bonds
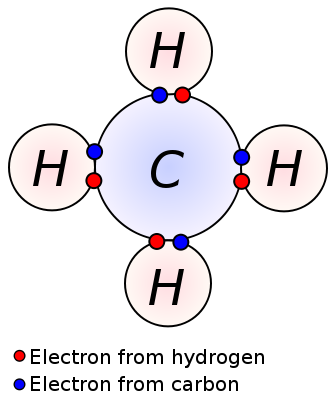
In case you haven’t realized it yet, graphene is completely made up of carbon. These carbon atoms are held together in their lattice by powerful covalent bonds, the same bonds used in diamonds. Because of these powerful bonds, graphene has a high melting point[3]. A key difference between the carbon atoms of diamond and the carbon atoms of graphene is their hybridization. Graphene has an sp^2 hybridization while diamond has an sp^3 hybridization. For our purposes, hybridization determines how many bonds carbon will have. Sp^2 means carbon will bond with 3 atoms and sp^3 means carbon will bond with four [4]. Carbon typically likes to have four bonds because it has four valence electrons. Graphene’s carbon atoms only use three out of their four valence electrons on bonds. What, you may be wondering, is this one, lone electron doing? Well, this electron is a free-floating electron and it grants graphene the ability to conduct electricity. This is a very important property. Sometimes, the free-floating electrons will bond with another, making the bonds within graphene even stronger. [3](Because even electrons want friends too…)
Covalent Bonds in Methane
Well, if graphene is so strong, why does graphite seem so weak? After all, graphite is just stacked graphene. Well, graphene doesn’t bond well when stacked. Instead of strong covalent bonds, graphene uses a weak and pitiful intermolecular force known as London dispersion forces [5]. Just about every molecule experiences these forces, and they are the reason why pencils can write. A good analogy is think of graphite as a stack of cards. The individual card is strong, but this does not translate to a durable stack of cards. Instead, they will slide off one another with just a gentle push.

Image Source
Is graphene useful?
Hopefully by now, you have a good idea of why graphene can be so strong but also so light and flexible. But, like my high school English teacher would say about my papers, “so what?” (I got a C in English that year). Is graphene useful? The answer is obviously yes. Graphene has many qualities which makes it useful in a broad range of subjects. Remember when I told you it was a good conductor. Graphene has a strong future in electronics. Studies show that it could be used for one of the most energy efficient transistors ever [6]. Others foresee graphene being used in flexible/foldable touchscreens [2]. It doesn’t stop at electronics. Because of graphene’s high melting point, there is talk of using graphene in the automotive industry which could save as much as 30% to 50% in automotive structural weight while also adding more safety to the operator.[7]. In biomedical engineering, there is talk of using graphene for tissue regeneration[8]. Honestly, the uses for graphene are limited only by our imagination.
Are we already using it? What are we using graphene for right now? Well, graphene is being used in censors. Think about it. Every atom of graphene is exposed to its environment [3]. When applied, graphene can make for incredibly accurate censors that can distinguish materials down to the molecule.
If you want to get a glimpse of the future, googling “potential uses for graphene,” is probably a good way to do it. Graphene is still a relatively new discovery, but I would classify it as a major discovery. I am excited to see what becomes of graphene and I hope you are too.
(Hey! Thank you for reading! I had a lot of fun researching and writing this post. If you have any feedback for me, you can comment below. That is always helpful and appreciated. My next post will be sometime this weekend. Thanks again for reading and I hope to see you again soon!
-ulearn)
Sources
http://www.mypearsonstore.com/bookstore/chemistry-structure-and-properties-0134293932
http://www.graphene.manchester.ac.uk/explore/the-applications/electronics/
https://www.chemguide.co.uk/atoms/structures/giantcov.html
http://chemed.chem.purdue.edu/genchem/topicreview/bp/ch10/carbon.php
https://en.wikipedia.org/wiki/Graphite
http://www.thegraphenecouncil.org/?page=ElectronicsJAN15
http://articles.sae.org/13441/
http://pubs.rsc.org/en/content/articlelanding/2013/tb/c3tb20405g#!divAbstract