The first photoelectrochemical experiments were carried out by the French scientist Antoine Becquerel in 1839 (Figure 1), who demonstrated the existence of an electric flow through the external circuit between two electrodes, by exposing a silver chloride electrode to the light Solar, producing in this way a photovoltaic effector that is known as the "Becquerel effect", the origin of this phenomenon was not understood until 1954 when Brattain and Garret, showed that the chemical reactions that occur on the surface of the germanium (semiconductor electrode) Could be influenced by the control of the semiconductor properties of germanium and also by the excitation of light, subsequently Gerischer in 1966, made detailed studies of electrochemistry and photoelectrochemistry of the semiconductor / electrolyte interface, however, it was not until after the crisis Of oil in 1973, when photoelectrochemistry had a frenetic period and despite several decades of research on this subject, there are still fundamental questions.
Fig. 1.- Illustration of the experiment realized by Antoine Becquerel (1839)
At present photoelectrochemistry can be defined as the electrochemical reactions produced by the incidence of photons in an electrode or on the solution, for the excitation of an electron from the valence band to the conduction band, producing in this way an electron / Hollow in the semiconductor producing a photocurrent, the excess energy is transmitted to the crystalline lattice through the thermal relaxation of charge carriers in the form of heat (photons). Two basic types of photoelectrochemical reactions can be distinguished as:
• Reaction on the electrode by photoexcitation, in this case a species in the solution could receive the excited electron or fill the hole with one of its own electrons. Figure 2 shows the excitation effect for a n-type semiconductor.
• Photoreactions of particles in solution. The reflection of the visible light of a particular frequency by the atoms or molecules of a solute is perceived by our eyes as the color of this substance.5 Under ultraviolet light, substances that do not absorb in the visible part of the spectrum can also be excited .
Additionally, also electron-hollow recombination can occur and excess energy can be converted to thermal energy; This recombination often proceeds through surface states and must be avoided.
Fig. 2.- Excitation process in an n-type semiconductor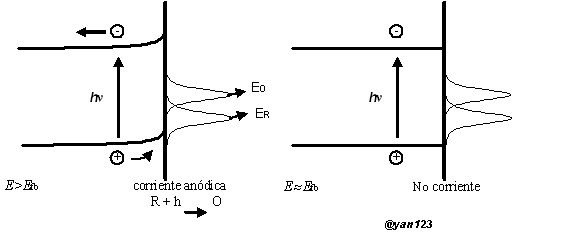
In current electrochemistry three types of photoelectrochemical cell can be found which in addition to the semiconductor electrode contain an auxiliary electrode and a reference electrode.
• Photovoltaic cells: as its name suggests, this involves the direct conversion of light to electric current. In principle, in these cells the compositions of the electrolyte do not change over time, in addition the reaction that occurs in the auxiliary electrode is the same as that in the semiconductor electrode.
• Photocatalytic cells: this cell has a similar operation to the previous one, nevertheless in this case the reaction is conducted in spontaneous direction, the energy of the light is used to overcome the prohibited band. This type of cell is widely used for substance conversion.
• Photoelectrolytic and photogalvanic cells: these cells involve the conversion of radiant energy into chemical energy for the conversion of substances. In the same way as the previous cell, ΔG> 0.
Fig. 3.- (a) photovoltaic cell, (b) photoelectrochemical cell, (c) photocatalytic.
Interesting post :)
Great contribution to the knowledge of science
i just upvoted. i find your content premium. thank you very much for such a big effort
Nice job. Could use a bit more in the citation department.