If we wish to get pure water from impure water, seawater for example, then we can get it through distillation process. Distillation is a method of separating mixtures to get pure liquid from impure one by heating up the mixtures, and condensate the water vapor to obtain pure water.
So the distillation method is based on differences in volatilities of liquid.
Distilled water is water that has had many of its impurities removed through distillation. Distillation involves boiling the water and then condensing the steam into a clean container. Source
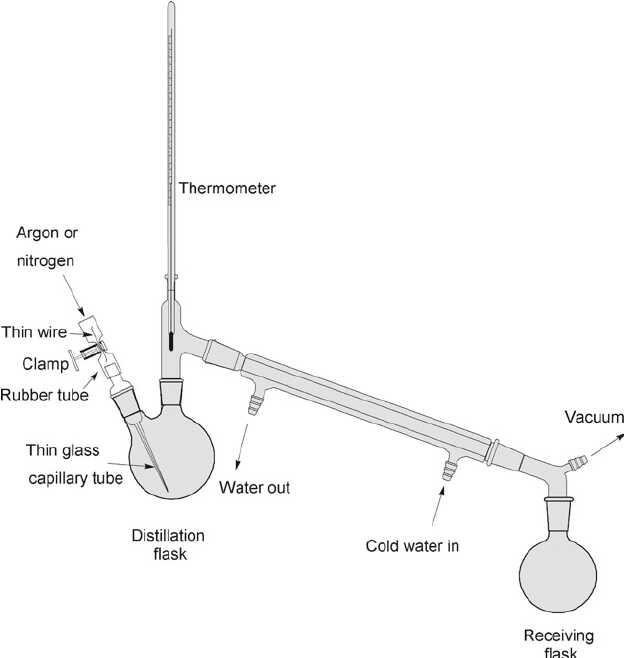
The work principles of a simple distillation ap is shown in Figure above. The water which is going to be distilled is poured on a heat-resistant flask, and then the flask is heated until the water is boiling and evaporating.
Then the formed water vapor is flowed away through a pipe. The pipe is already equipped with a condenser filled with cold water which flow in the opposite direction with the steam.
As a consequence, the pure water vapor which is in contact with the cold water condensate, resulting grains of pure water that will increase in process of time. Then the pure water is accumulate in a glass or a flask.
We can also use the distillation apparatus to get pure alcohol from the mixture of alcohol and water. The boiling point of alcohol is lower than water, so the formed steam mostly contains of alcohol vapor, so we obtain pure alcohol from the distillation process.
Sources :
laboratory tutorials of distillation