Distillation methods
In practice, distillation can be carried out by two main methods. The first involves producing a vapor by boiling the liquid mixture to be separated, then condensing these vapors, without bringing any portion of the condensed liquid as reflux to the reboiler so that they come into contact with the vapor that falls off. In the second method, a part of the condensate is sent to the reboiler in such a way that this return is in intimate contact and in countercurrent with the vapors that are released and go towards the condenser. This last method is so important that it has received the special name of rectification.
Equilibrium distillation.
There are two important types of distillation that do not involve rectification; the first of these is the so-called distillation of equilibrium or flash distillation and the second is that of simple distillation or differential distillation. Flash distillation involves the vaporization of a given fraction of a liquid charge, maintaining both the liquid and the vapor formed, in intimate contact until the end of the operation, so that the vapor is always in equilibrium with the liquid, removing the vapor and condensing it. The relationship between the liquid and vapor compositions at the end of the process are those that correspond to the equilibrium diagram of the distilled mixture.
source

Flash distillation is not of great importance in the distillation of two-component systems. This method is used with multicomponent systems, such as oil refining, where the mixture that is oil is heated under pressure in exchangers formed by tubes, the pressure is lowered and the heated liquid is vaporized by the sudden drop in pressure in conditions approximated to those of equilibrium with the superheated liquid.
Differential distillation.
In the case of differential distillation or simple distillation, the vapor generated by boiling the liquid separates as it forms from contact with it and then condenses.
The distillation, simple or differential, approximates the discontinuous commercial processes in which the steam is eliminated as it is formed without appreciable condensation. However, this process is not effective as a separation method, especially in the case where the components to be separated have their boiling points far apart and the methods that produce clearly defined separations are not necessary even when it is possible to use them. Also, the laboratory distillations that are carried out without reflux columns correspond to this type of distillation.
source
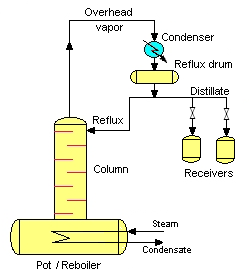
Rectification.
Rectification is the most used in practice as a separation method and is the one that has received more development. In the following figure, a rectification unit is schematically represented.
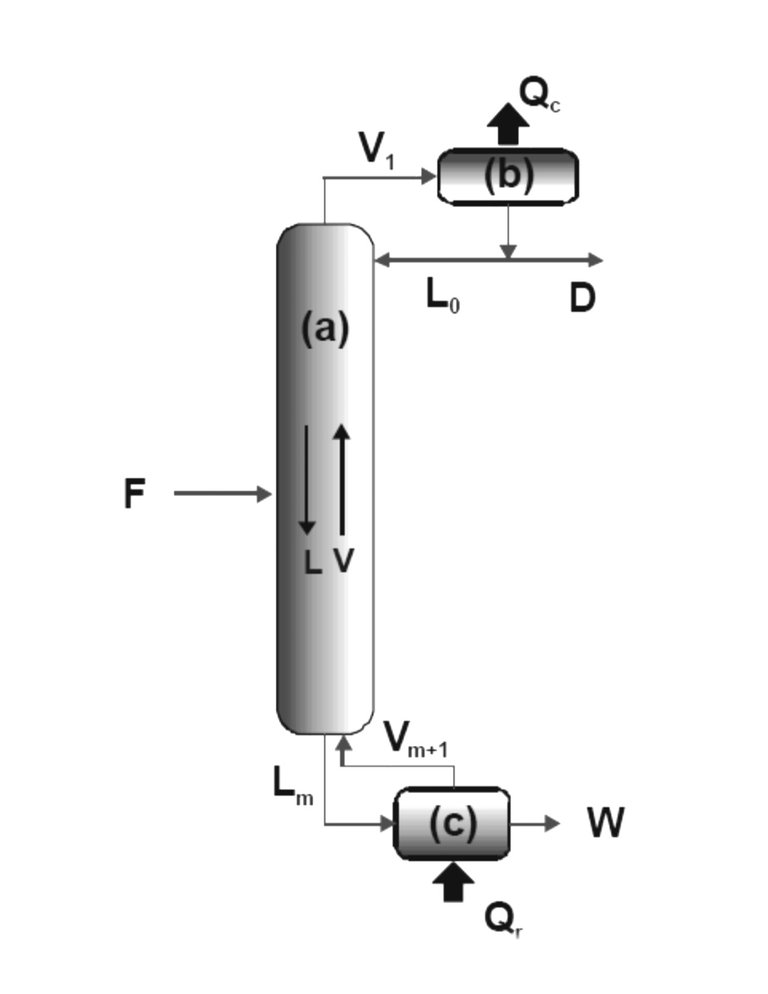
a) A column of rectification or fractionation through which the vapors rise to contact in countercurrent with the liquid that descends.
b) A condenser that condenses the vapors that come out of the upper part of the column, sending part of this condensed liquid (reflux) back to the column so that it falls in countercurrent with the rising vapors, and discharging the rest of the liquid as a Distilled product.
c) A reboiler (or reboiler) in which steam is generated.
Currents:
F: Feeding to the column.
V1: Steam coming out from the top of the column.
L0: Liquid that returns (reflux) to the column.
D: Distilled.
Lm: Liquid that comes out of the bottom.
Vm + 1: Steam that returns to the column.
W: bottom product.
L: Current of liquid that goes down the column.
V: Steam current that rises through the column.Heat exchange:
Qc: heat that is extracted from the condenser.
Qr: heat delivered to the reboiler
As the liquid descends through the column, it is enriched in the components of the mixture that have the highest boiling point and as the vapor current rises through the column it is enriched in the components of the lower boiling point. Consequently, the column is an appropriate device to put these two currents in close contact, in such a way that the vapor current tends to vaporize the components of the low boiling point that carries the liquid stream, and this, in turn, tends to Condensate the high-boiling components that carry the vapor stream. The head or upper part of the column is colder than its base or bottom so that the stream of liquid warms up as it descends and the vapor stream cools as it rises. This transmission of heat is carried out by contact between the liquid and the vapor, and for this, it is required that there is a very intimate contact.
The words "fractionation" and "rectification" are used with great confusion and different meaning. Fractionation is an old term that was born to designate distillation methods that were imperfect than the current ones. However, the name has persevered in the oil industry in which the columns are almost always known as "fractionation columns"; On the other hand, the same process in many industries is known as rectification, a term that was born in the alcohol industry. It also leads to increased confusion, that the entire process that is verified in the columns, can be called "rectification", but the same word can be used with a more concrete meaning, to designate only the part of the column that is above of the feeding dish. Consequently, it is a matter of local custom that the entire column be called a "fractionation column" or a "rectification column".
References:
Very educational your post, I really like the subject of distillation, I invite you to read my last post that talk about the fractionation in a debutanizing tower.
Good :)