Science Worksheet For Junior High School
Osmosis is the movement of water molecules through the semipermeable membrane from the more dilute part to the more concentrated part. The semipermeable membrane used must be penetrated by the solvent (in this case water), but it can not be penetrated by the solute. The process of osmosis is a natural phenomenon, but can be artificially inhibited by increasing the pressure on the more concentrated so that the more dilute part.
Osmosis process is what causes water, oxygen or carbon dioxide can easily pass through the cell membrane in plants. The molecules will diffuse from areas of high concentration to areas with lower concentrations. This process will stop if the concentration of substances on both sides of the membrane is balanced.
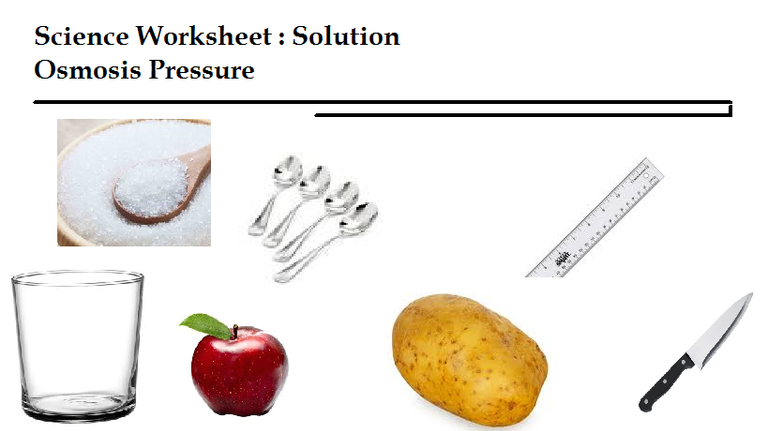
Tools and materials
Tool:
- Knife
- Ruler
- Scales
- Glass
- Spoon meal
Ingredients:
- Aquades
- Sugar
- Salt kitchen
- Apple
- Potatoes
Work procedures
- Fill 6 pieces of glass with aquadest of 50 mL
- Add 1 tablespoon of salt to the glass no 3 and 4, and 1 tablespoon sugar on the glass 5 and 6. Stir until dissolved.
- Slice the apple into 3 cube-shaped sections with a size of 2 cm x 2 cm x 2 cm. Do the same for potatoes.
- Weigh each piece of fruit
- Insert each piece into the glass containing the solution.
- After 30 minutes, take the pieces of fruit then measure the fruit pieces earlier and also the mass.
Data Observation
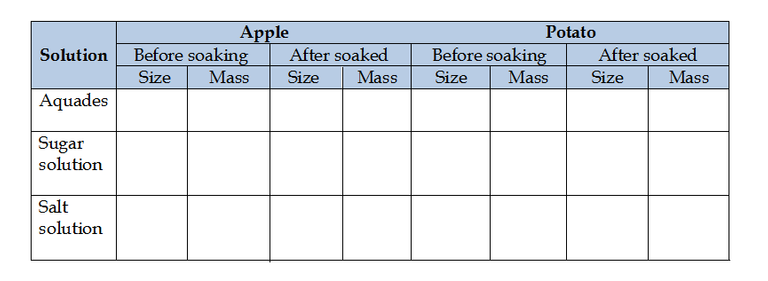
Question
- From the experiment, how is the diffusion of water in each experiment?
- Try using some other types of fruit (eg carrots), whether it will produce the same results.
- Make a trial report and present the results in front of the class.
What is conclusion??
Lets learn more about science more....
Thanks for coming here...