Heat and Temperature
When you rub your hands together, they become warm. While touching an ice, hands become cold. During aeroplane crash, it becomes so hot that it catches fire. In all these examples, the body is becoming hot or cold by a process.
When the body becomes hot, it shows certain physical characteristics like vapour comes from hot tea, the hot iron rod becomes red etc. But, to observe what's really going on, we have to go in molecular level. The molecules are in random motion in bodies. In cold bodies, the movement of molecular is slow whereas, in hot bodies, the molecules are in rapid motion. From this concept, let us try to quantify heat and temperature.
The energy associated with the motion of the object is called kinetic energy.
Kinetic Energy
Now, add all the kinetic energy of molecules present, the sum is what we call heat energy. If we take the average value of kinetic energy of molecules, we call it temperature.
Example
Consider in Jar 1, there are 4 molecules with kinetic energy 50 J, 60 J, 40 J, 50 J and consider another Jar 2, let there be 2 molecules with kinetic energy 50 J and 100 J. Let us calculate heat and temperature of each jar. J here represents Joule, SI unit of energy and K here represents Kelvin, SI unit of temperature.
For Jar 1
Heat = sum = 50+60+40+50 = 200 J
Temperature = proportional to Average = (50+60+40+50/4) = 50 K
For Jar 2
Heat = sum = 100+ 50 = 150 J
Temperature = Average = (100+50/2) = 75 K
The heat energy in Jar 1 is greater than heat energy in jar 2.
The temperature of Jar 1 is less than the temperature of jar 2.
This suggests that higher heat doesn't necessarily imply higher temperature.
Analogy
Imagine two liquid vessels with water filled at a different level. In this example, the analogy of heat is the amount of water and analogy of temperature is the height of water level above ground.
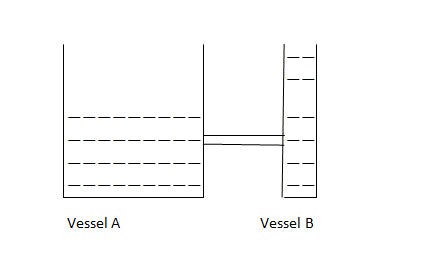
i. Which vessel has more amount of liquid?
It is vessel A, so it represent object with larger heat.
ii. In which vessel, water level is higher?
It is in vessel B, so it represents objects with high temperature.
If we connect pipe and allow flow of water, the water will flow from vessel B to vessel A until level of water becomes same in both vessel.
Equivalently, when two objects at different temperatures are made in contact with each other, the heat flows from body at higher temperature to body at lower temperature until temperature of both object become same. This condition is called thermal equilibrium.
References
- Concepts of Physics, HC Verma.
Hi! I am a robot. I just upvoted you! Readers might be interested in similar content by the same author:
https://steemit.com/steemstem/@riemannian/heat-and-temperature
.