Faraday's Laws of Electrolysis
The relationship between the quantity of electric charge passed through an electrolyte and the amount of the substance deposited at the electrodes was presented by Faraday in 1834, in the form of laws of electrolysis.
(i) Faraday's First Law
When an electric current is passed through an electrolyte, the amount of substance deposited is proportional to the quantity of electric charge passed through the electrolyte.
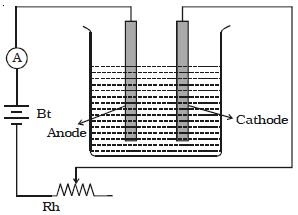
If W be the mass of the substance deposited by passing Q coulomb of charge, then according to the law, we have the relation:
W ∝ Q
A coulomb is the quantity of charge when a current of one ampere is passed for one second. Thus, amount of charge in coulombs,
Q = current in amperes × time in seconds
= 1 × t
So W ∝ 1 × t
or W = z × 1 × t
where z is a constant, known as electro-chemical equivalent, and is characteristic of the substance deposited.
When a current of one ampere is passed for one second, i.e., one coulomb (Q = 1), then
W = Z
Thus, electrochemical equivalent can be defined as the mass of the substance deposited by one coulomb of charge or by one ampere of current passed for one second. For example, when a charge of one coulomb is passed through silver nitrate solution, the amount of silver deposited is 0.001118 g. this is the value of electrochemical equivalent of silver.
(i) Faraday's Second Law
When the same quantity of charge is passed through different electrolytes, then the masses of different substances deposited at the respective electrodes will be in the ratio of their equivalent masses.
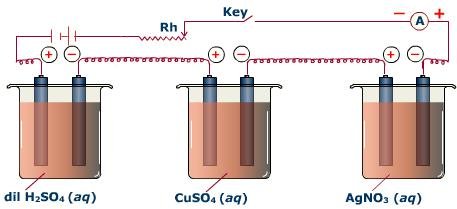
The law can be illustrated by passing same quantity of electric current through three voltametres containing solutions of H2SO4, CuSO4 and AgNO3 respectively as shown in Fig.12.1. In the first voltameter, hydrogen and oxygen will be liberated; in the second, copper will be deposited and in the third, silver will be deposited.
(Mass of hydrogen)/(Mass of copper) = (Equivalent mass of hydrogen)/(Equivalent mass of copper)
or (Mass of copper)/(Mass of silver) = (Equivalent mass of copper)/(Equivalent mass of silver)
or (Mass of silver)/(Mass of hydrogen) = (Equivalent mass of silver)/(Equivalent mass of hydrogen)
It is observed that by passing one coulomb of electric charge.
Hydrogen evolved = 0.00001036 g.
Copper deposited = 0.0003292 g.
and Silver deposited = 0.001118 g
These masses are in the ratio of their equivalent masses. From these masses, the amount of electric charge required to
deposit one equivalent of hydrogen or copper or silver can be calculated.
For hydrogen = 1/0.0001036= 96500 coulomb
For copper = 31.78/0.0003292= 96500 coulomb
For silver = 107.88/0.001118 = 96500 coulomb
This follows that 96500 coulomb at electric charge will deposit one g equivalent of any substance. 96500 coulomb us termed as one Faraday and is denoted by F.
Again according to first law,
W = Z×Q
Then Q = 96500 coulomb, W becomes gram equivalent mass (E).
Thus, E = Z×96500
or Z = E/96500
z1/z2 = E1/E2
Fundamental unit of charge:
As one g-equivalent of an ion is liberated by 96500 coulomb, it follows that charge carried by one g-equivalent of an ion is 96500 coulomb. If the valency of an ion is 'n', then one mole of these ions will carry a charge of nF coulomb. One g-mole of an ion contains 6.02 × 1023 ions.
Then,
The charge carried by an ion = nF/(6.02 × 10^23 ) coulomb
For n = 1,
The fundamental unit of charge = F/(6.02 × 10^23 )
i.e., 96500/(6.02 × 10^23 ) = 1.6 × 10^-19 coulomb
or 1 coulomb*=6.24 × 10^18 electrons
The rate of following of electric charge through a conductor is called the electric current.
Coulomb:
It is the unit of electric charge. It is the amount of charge that moves past may given point in a circuit when a current of 1 ampere is supplied for one second.
1 coulomb = 1 ampere - second
It is also defined as the amount of charge which is required to deposit by electrolysis 0.001118 g of silver from a solution of silver nitrate.
An electron has 1.6×10^-19 coulomb of negative charge. Hence, one coulomb of charge is carried by 6.24 × 10^18 coulombs. 1 mole of electrons carry a charge of 96500 coulomb. This quantity of charge is called Faraday.
Charge carried by 1 mole of electrons
= (6.023×10^23)(1.6×10^-19)
= 96368 coulomb
= 96500 coulomb
References:
Hi! I am a robot. I just upvoted you! I found similar content that readers might be interested in:
http://www.sakshieducation.com/(S(0lasgpi3ii5mkb55aphxvzjw))/Inter/Material/IIndYearEM/Chemistry/03_04_Faradays_Laws_of_Electrolysis_and_Applications.pdf
It is not true.
check again