This is an educational post, intended to be funny, not some fake news.
The direct inspiration for this post came to me after reading the comment from some concerned Steemian, who was wondering what if we create antimatter and destroy the world.
Let's do some funny calculations!
What chemical elements build our bodies?
As you can see from this infographics I just stole from Wiki, 99% of our bodies consist of 11 elements.
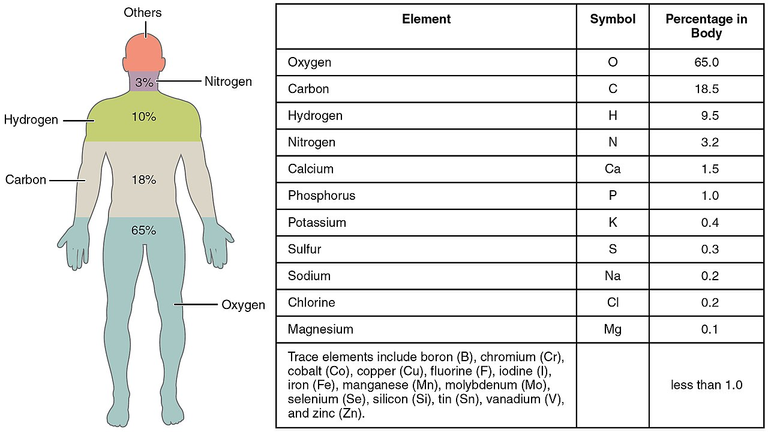
And, some elements have isotopes that are not stable.
For our calculation, oxygen is not interesting, because it has 3 isotopes, all of them stable.
The next most abundant element has 3 natural isotopes + 1 synthetic. One of them is 14C, that is radioactive, Beta(-) decay. Yes, that 14C, used in carbon dating. While we are alive, we ingest the carbon in all its forms, and when we stop being alive, the amount of unstable carbon decreases.

Not this kind of dating... source
Next element is Hydrogen, with 3 isotopes, and one of them is Tritium, that undergoes Beta(-) decay.
Some other elements, not that much present in our bodies also contribute to the radioactivity of... Us...
If you take a closer look, you could see that we have about 200 mg of radioactive isotopes!
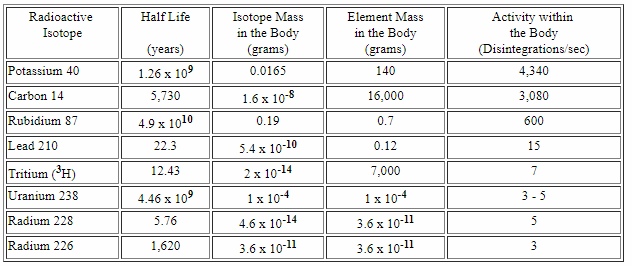
Source
Let's check the decays now
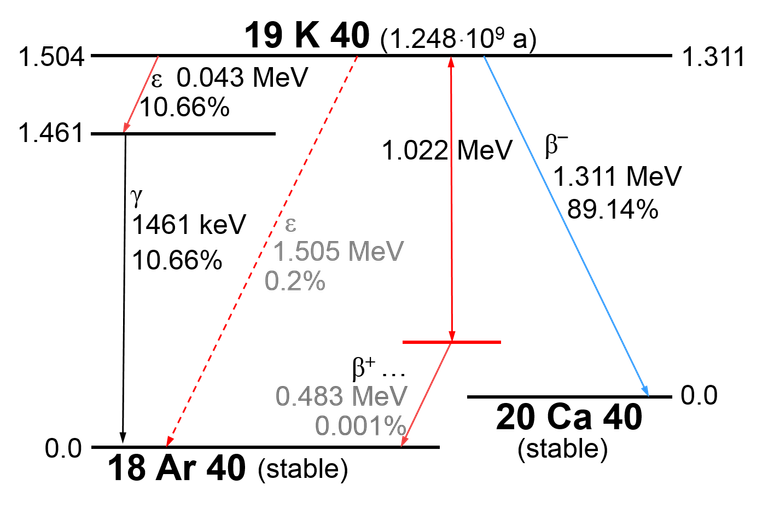
The majority of radioactive events in our body comes from 40K:
You see that Beta(-) at the right side of the image?
That is particularly interesting because Beta(-) decay goes like this:
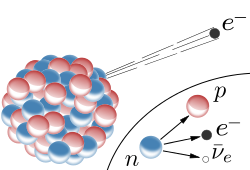
Thanks again, Wiki
You can easily recognize the markings, e - electron, p - proton, n - neutron and some strange letter ν with a dash?
That is the mark for anti-neutrino, yes, you are producing antimatter!
And rarely, it will decay to 40Ar by emmiting positron and neutrino.
And positron = anti-electron
The mechanism for carbon fourteen is basically the same:
The third source of our radioactivity is 87Ru, Rubidium, the element without the function for us, but it's abundant and we contain it, just by chance. It also decays via Beta(-) decay.
I will skip several elements because they are also decaying by emitting Beta particles.
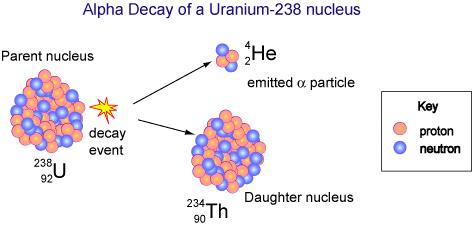
Uranium-238 is a bit more interesting because it decays by emitting alpha particles:
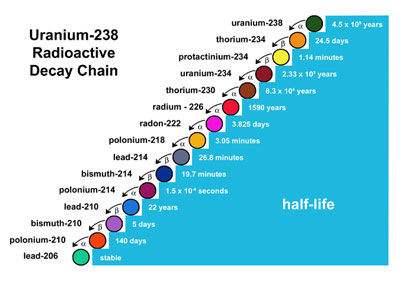
Uranium-238 is a restless soul and it continues to decay, on and on...
One of those elements from the cascade is also present in the table above, and it's the Radium 226.
But, how much radioactivity is this?
Well, about 1/10 of the total radiation we experience per year, comes from our own bodies (about 0.3 mSv per year), source
And how much antimatter do we produce?
According to Symetry Magazine, banana pop-up the positron every 75 min form atypical potasium Beta(+) decay.
And the banana contains about 400 - 500 mg of potassium while we have about 140 gr it means that we produce 3-4 positrons every minute. Not bad!
Next time someone says you are not productive, listen and answer: "I just made a positron ..."
...and get fired with style.
References:
- Symetry Magazine
- Table
- And lot of half-sentence facts, linked in text


excellent post friend, greetings already voted
Thank you geologist ;)
Pro tip: Don't explain to the TSA that you are irradiating the airport by your mere presence :D
:D
Hm, interesting. But has anyone tried ingesting a lot of bananas? It might just be a matter of reaching a critical mass before disaster strikes.
(grabs bananas) TO THE LAB !!!!
Quite informative. I like your mode of communicating the topics.
Nice work. Keep in coming
That was a good read. I didn’t know, well, any of that before! 🤓