Hi, today i want to share with you an experimental synthesis to produce aspirin, but before that there is a small introduction to know better what is aspirin and its history.
Source: (https://www.express.co.uk/life-style/health/874047/aspirin-wonder-drug-reduces-risk-of-dementia-cancer-stroke-heart-attack)
source: (https://theodora.com/drugs/bayer_aspirin_tablets_bayer_healthcare_llc.html)
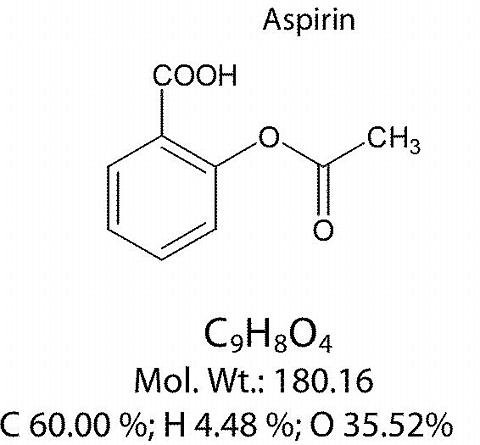
- it is one of the best-selling drugs in the world, aspirin is the trade name of acetylsalicylic acid, it is synthesized by esterification between acetic anhydride and salicylic acid.

Source: (http://www.luzhongchemical.com/acetic-anhydride/acetic-anhydride-c4h6o3-with-a-purity-of-99.html)

Source: (https://acne.ooreka.fr/astuce/voir/447865/acide-salicylique)
HISTORICAL:
From the Greeks, decoctions of leaves and willow bark were recommended to combat fever and pain.
Source: ( )
)In 1830, Leroux isolated the active ingredient and named saliciline. In 1860 Kolbe synthesized salicylic acid from phenol and carbon dioxide. But this acid is bitter and poorly tolerated at the gastric level.
Aspirin or acetylsalicylic acid is manufactured and marketed for the first time in 1899 by the German firm Bayer.
because of its properties, it is one of the most consumed drugs in the world.Synthesis (practical):
1- Goal: the purpose of this protocol is to achieve a step of industrial synthesis of aspirin and purify the product by recrystallization.experimental protocol:
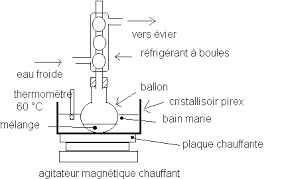
1st Step:
A- Heat the water bath 50 ° C.
B- Introduce in a 250ml balloon (dry):
- 5g of salicylic acid.
- 7 ml of acetic anhydride.
- 3 drops of concentrated sulfuric acid
- A magnetic bar.
C- Fit a water cooler on the flask, turn on the water circulation and place the flask in a water bath for 20 minutes.
D- Switch on the magnetic stirrer.
2- Cooling:
- Remove the flask from the water bath, cool it under a stream of cold water.
- Add slowly 60ml of ice water.
- Then olace the balloon in an ice-water bath for about ten minutes.
- Meanwhile, cut 2 filters to the size of the Buchner.
- filter on buchner, and rinse the flask with ice-cold distilled water, then rinse gently.
- Place the resulting solid in an Erlenmeyer flask, and crush the lumps with a glass stirrer.
finally, a solid is obtained which impure acetylsalicylic acid.
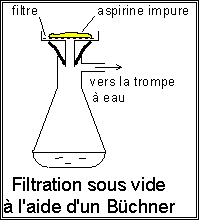
Purification and recrystallization:
The purpose is to purify acetylsalicylic acid using the difference in solubility between a body and its impurities in a solvent.
The impure acetylsalicylic acid is dissolved in a suitable solvent.
- When the solution cools, the aspirin crystallizes and the impurities remain in solution.
The equation of the reaction associated with the synthesis of aspirin:
C6H6O3+ C4H6O3-------------- C9H8O4+ C2H4O2.
- The role of sulfuric acid:
sulfuric acid is a catalyst.
The benefits of aspirin:
decreased pain.
decrease in fever.
anti-inflammatory.
prevents blood clotting.
Like all drugs, aspirin has risks and contraindications, studies indicated that a daily intake was accompanied by a risk of gastrointestinal bleeding.Then you should consult a doctor first in case of chronic pain.
Réferences:
Cours of organic chemistry, university of mostaganem, Faculty of Exact Sciences and Computer Science, Department of Materials Science (2012).
https://fr.wikipedia.org/wiki/Acide_ac%C3%A9tylsalicylique
http://www.aspirine.fr/historique.html
http://www.e-sante.fr/aspirine
This post is worth hundreds of dollars. you have done a great deed. Only through this valuable knowledge, can we learn the truth.
thank you i appreciate that you find time to read this post :)
I am a nurse; this was a pretty interesting post- just because of my sheer interest in medicine. It’s cool to see stuff on here that is in my world!
thank you :) i'm glad that you like this post
Interesting, good job 👍
thank you :)
very informative, thank you for sharing your knowledge
you welcom, thanks for reading :)
Great job @benainouna :)
thank you my master :)