
We all know how to spark the fire (I’m not referring to Gwen Stefani's terrible song) .
You take something to burn, make some heat, add some oxygen and you got yourself a chemical reaction called combustion!
OK, that’s great, we have fire now. But what is actually fire? What are flames made of? And why do different fuels produce different flame colors?
The Answer
During the combustion, atoms inside the burning material get mixed with the oxygen in the atmosphere creating new molecules. In the process the atoms get excited and release energy in form of light.
The wavelength emitted carries information about the burning material, in other words you can tell what is burning by the color of the flame.
Flames also tend to go up and that’s because how gravity works. If you light a match in microgravity, like on the ISS, this is how a flame will look like.

But flames have another interesting characteristic: Flames are made (in part) of Ions.
Ions are charged atoms which react in a magnetic environment, in fact flames can be manipulated by an electric field making the flame deflect according to the polarity of the electric field just like in the picture below.

Source
If you have a Random Question to ask, just comment below and wait for the next post!
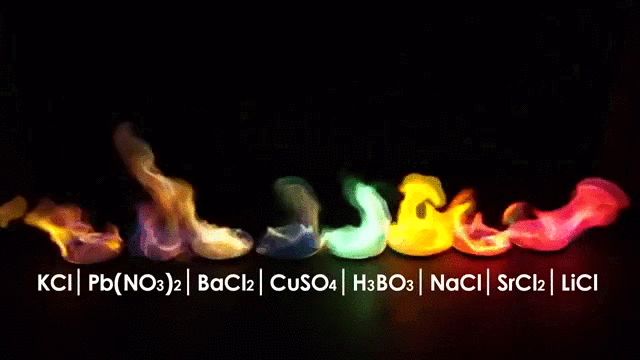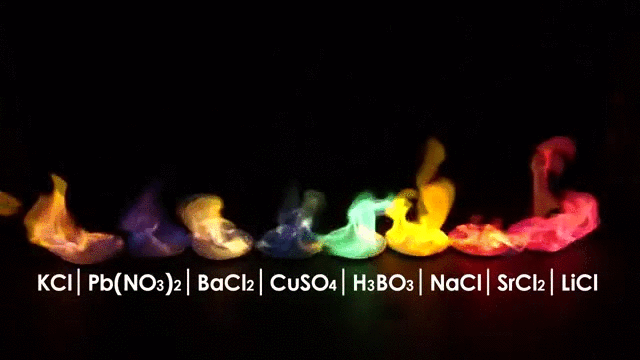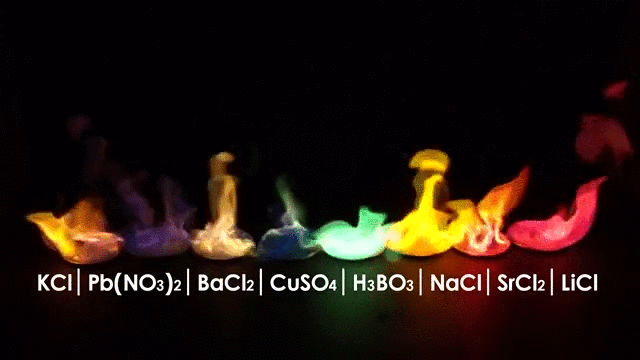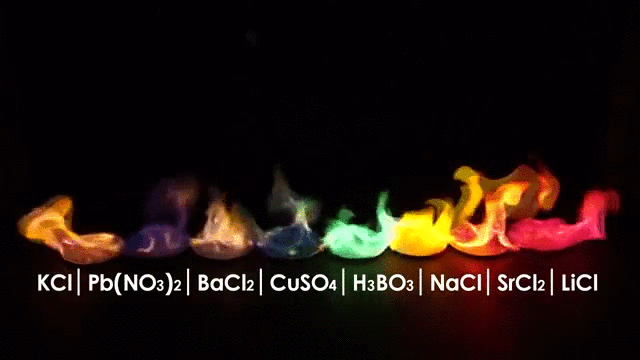
Nice post and great job with the image citations. However the post could also use some information citations too.
Thanks for reading and thanks for the tip, on the next post I'll provide links to the resources I've consulted.
nice post
Thanks!
This post has received a 3.36 % upvote from @booster thanks to: @bendelgreco.
This post has received a 0.49 % upvote from @buildawhale thanks to: @bendelgreco. Send at least 1 SBD to @buildawhale with a post link in the memo field for a portion of the next vote.
Steem WitnessTo support our daily curation initiative, please vote on my owner, @themarkymark, as a