Introduction
Iron (Fe)
Symbol: Fe
Atomic Number: 26
Atomic Weight: 56
(pixabay.com)
It is second most abundant metal in the earth crust. It exists in two valencies i.e. +2 and +3. It exists in the form of different ores which are following:
Magnetite (
 )
)Hematite (
 )
)Siderite (
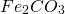 )
)Iron pyrite (
 )
)
Extraction
Following metallurgical processes are carried out for the extraction of Iron from its ore Hematite ( )
)
1. Crushing and Pulverizing
In this process big lumps of ores are taken out from the mine are crushed to gravel form using jaw crusher and grinded to a fine powder using ball mill.
2. Concentration
Since the ore is an oxide in nature, concentration can be carried out either by magnetic separation or by gravity separation. In this process, crushed ore is charged into gravity separation tank through funnel and current of water is passed from the bottom. The ore is agitated by water. During the process, heavier ores gradually deposited at the bottom and lighter impurities are swept away by the current of water.
3. Calcination of Ore
In this process, the ore is heated with the limited supply of air. During the process, the impurities presence is removed as their volatile oxide.
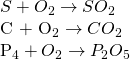
Moisture present is removed as their vapour. Similarly, if ore contains FeCO3, it also gets oxidize to Fe2CO3

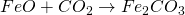
4. Reduction of Ore
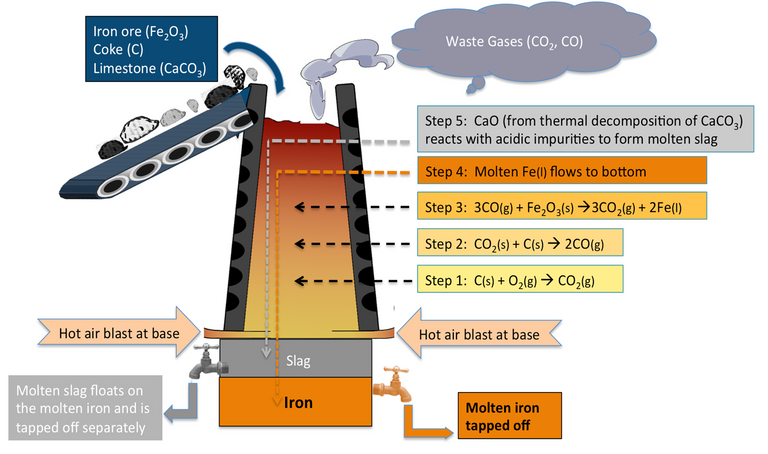
In this process, the calcinated ore is mixed with coke and CaCO3 in the ratio 8:4:1 and heated with pre-heated air in a special device called Blast furnace.
The following changes take place inside the blast furnace:
i. Zone of preparatory heating
The temperature of this zone ranges from 500-600 ℃ and in the zone water present in the ore is removed as water vapour and impurities if present is removed in volatile form. (oxide)
ii. Zone of reduction
The temperature of this zone ranges from 600-800 ℃ and in the zone reduction of hematite ore by CO takes place as:
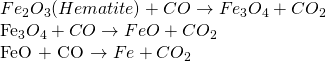
iii. Zone of slag formation
The temperature of this zone ranges from 800-1000 ℃ and in this zone CaCO3decomposes to give CaO which acts as the basic flux and combines with acidic impurities to form the fusible mass called slag.
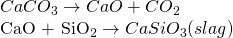
iv. Zone of fusion
The temperature of this zone ranges from 1000-1200 ℃. In this zone, iron gets melted and the molten iron absorbs some other impurities like carbon, Si, Mn, P, etc.
v. Zone of combustion
The temperature of this zone rises to 1300℃ and above. In this zone, coke combines with oxygen to form CO2.

The reaction is exothermic in nature. CO2 so formed readily combines with C to form CO.
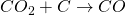
The reaction is endothermic in nature. As CO2 rises up it comes in contact with more carbon and forms CO. As the reaction is endothermic in nature; the temperature in blast furnace decreases as one moves upward. Now the molten iron forms lower layer on the hearth of furnace and slag forms the upper layer of the hearth of the furnace which is taken out through the respective outlet.
Physical properties
Pure iron is white, lustrous but impure iron is gravy in nature.
Highly malleable and ductile.
Good conductor of heat and electricity.
Chemical Properties:
i. Action with air:
No action with dry air but it reacts with moist air.
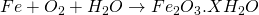 (Reddish brown solid called rust)
(Reddish brown solid called rust)
When Fe heated with air it forms Ferric oxide.
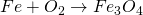
ii. Action with water:
No action with pure and cold water. It reacts with steam and forms hydrogen gas.
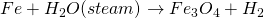
This process is called lane process. It is used in the manufacture of H2.
iii. Action with CO
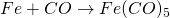 (Ironpenta-carbonyl)
(Ironpenta-carbonyl)
It is used in making magnetic tape.
iv. Action with acid:
a. Action with H2SO4

b. Action with hydrochloric acid

c. Action with HNO3

When Fe reacts with concentrated HNO3 non-reacting metal oxide i.e. Fe3O4 first formed get deposited on the surface of iron and contact of Fe with NO3 breaks down. Hence, the reaction gradually slows down and stops. This property is called passivity of iron. This protects inner layer of iron.
Uses
It is used in the manufacture of steel.
It is used in civil engineering structures i.e. Reinforced Concrete, Girders etc.
It is used to make metal cutting tools, cylinder block of the piston, automobile parts etc.
References
i. Foundations of Chemistry, Moti Kaji Sthapit
ii. Conceptual Chemistry, SK Jain
iii. College Chemistry, Ganesh Dahal
Nice article on Iron bro😀
When potassium and nickel is used with iron, we can manufacture a knife😀
Potassium - K
Nickel - Ni
Iron - Fe
heheheh really funny bro.
Thanks,
This post has received a 2.2 % upvote from @boomerang thanks to: @bikkichhantyal
Good article mate! I had no idea pure iron was white, I always assumed it was just... grey!
Thank you mate. One upvote would have encouraged me to write these posts in future too. :D