Hello steemains, how are you? hope you guys are fine. I am going to start new series of post about laws of chemical combination from this article.
During seventeenth century, scientists performed various experiments to observe the relationship between reactants and products in chemical combinations. They studied about quantitative relationship like mass-mass, mass-volume, volume-volume relationships and formulated laws.
The branch of chemistry which deals with quantitative measurement and relationship between products and reactants in a chemical reaction is called Stoichiometry.
The quantitative relationship in chemical reaction is governed by five laws of chemical combination
i) The law of conservation of mass
ii) The law of definite proportion
iii) The law of multiple proportions
iv) The law of reciprocal proportions
v) The law of gaseous volume
Law of conservation of mass
This theory was first put forward by Russian scientist Lomonosov in 1748 AD. Big trees grow out of tiny seed. When water boils, it weighs less than before. It seems that the mass is created in first example whereas mass is destroyed in second example. Actually, the seed gets the matter from nutrition; air etc so it grows bigger. In second case, the water is changed into vapor. It changes state but matter isn’t destroyed. In no chemical reaction, matter can be created or destroyed. It is called conservation of mass.
It can also be stated as "The total mass of the reactant consumed is equal to the total mass of product formed."
Consider reaction in which reactants A and B react to form C and D as product in isolated condition. Let mass of A be a, B be b, C be c and D be d in grams. Then,
Total mass of reactant = Total mass of product
Or, a+b = c+d
Later, Einstein showed that mass is the concentrated energy. Mass and energy can be inter-converted. They are the same form of matter.
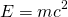
m is the mass whose equivalent energy is E. c is the speed of light in vacuum.
This principle is called mass energy equivalence.
So, the law can be stated as “total amount of mass and energy of the system remains same during a chemical reaction’’.
The above law can be illustrated using following experiments:
Lavoisier experiment
Lavoisier performed the original experiment to verify this law. He heated the tin in a closed vessel. The weights before and after the reaction were same. This verifies the law.
Tin + Oxygen → Tin Oxide
Charcoal experiment
A round bottom flask is provided with a tightly fitting stopper, through which two stout copper wires are introduced. To the end of one of the wires is soldered a small capsule made up of copper. A piece of charcoal is placed in the capsule and a thin Platinum wire joining two copper wires at their ends, is wound round in it. The flask is now filled with oxygen by displacement of air. The stopper is replaced and tightly fitted, and the whole apparatus is carefully weighed. Electric current is passed through the platinum wire which becomes red hot, the piece of charcoal burns away to form carbon dioxide gas. At the end of experiment, whole apparatus is weighed again. The weight remained unchanged though the charcoal disappeared. This proves law of conservation of mass.
Carbon + Oxygen → Carbon dioxide
Landolt’s experiment:
Landolt took solutions of Silver nitrate and sodium chloride in two arms of H-shaped tube called Landolt’s tube as shown in figure. It is carefully weighed. By tilting, solutions were carefully taken in contact so that they can react. Curdy white precipitation of silver chloride was obtained due to reaction of silver nitrate and sodium chloride. After the reaction was over, the vessel was weighed again. The weight was practically unchanged. This proves the law of conservation of mass.
Silver Nitrate + Sodium Chloride → Silver Chloride + Sodium Nitrate
Examples:
Example 1
100 g of calcium carbonate was heated so that there is no further loss in weight. The final mass was found to be 56 g. How much carbon dioxide was given out?
Solution:
The chemical reaction is
Calcium carbonate → Calcium Oxide + Carbon dioxide
Mass of calcium carbonate = 100 g
Mass of calcium oxide = 56 g
Mass of Carbon dioxide = x(let)
From conservation of mass
100 = 56 + x
Or, x= 44 g
So 44 gram of carbon dioxide was given out.
Example 2
When 4.2 g of sodium bicarbonate is added to a solution of acetic acid weighing 10 g, it is observed that 2.2 g of Carbon dioxide is released to the atmosphere. The residue left is found to weigh 12.0 g. Show that these observations are in agreement with conservation of mass.
Solution:
Mass of reactant = 4.2 + 10 = 14.2 g
Mass of Product = 2.2 + 12 = 14.2 g
Here,
Mass of Reactant = Mass of Product
So, these observations are in agreement with conservation of mass.
References
[chemistry-reference]http://chemistry-reference.com/problems/question_1.asp)
Steemstem:
SteemSTEM is a community driven project which seeks to promote well-written and informative Science, Technology, Engineering and Mathematics posts on Steemit. The project involves curating STEM-related posts through upvoting, resteeming, offering constructive feedback, supporting scientific contests, and other related activities.
DISCORD: https://discord.gg/j29kgjS

Wow thanks for reminding me these basis....... Science / Chemistry is always fun
you are welcome. :D :D
Thats simply amazing i really dint know about these basics..
Thank you.
I am glad that you know some basics now :D :D
Thanks for your sharing this, can you also re-new discord link please? Also you all everybody can vote for me as a witness via clicking here
done. thanks.
Why should we vote you as witness?
Please give some info about you and your contribution to steemit.